Unraveling The Threads Of Ionization Energy: Trends And Insights In 2025

Unraveling the Threads of Ionization Energy: Trends and Insights in 2025
The year is 2025. Advances in quantum computing and experimental techniques have propelled our understanding of atomic and molecular interactions to unprecedented heights. As we delve deeper into the intricacies of matter, a fundamental property continues to hold immense significance: ionization energy. This quintessential measure of an atom’s ability to resist losing an electron remains a cornerstone of chemistry, guiding our understanding of chemical bonding, reactivity, and the very essence of matter itself.
This article explores the latest developments in understanding periodic trends in ionization energy, highlighting the interplay between theoretical frameworks and experimental observations. We will delve into the intricate dance of factors influencing ionization energy, from the influence of nuclear charge and electron shielding to the fascinating role of electron configuration and orbital penetration. We will also explore the exciting frontiers of research in this field, including the potential for manipulating ionization energy through external stimuli and its impact on cutting-edge technologies like solar energy and quantum computing.
Understanding the Fundamentals: A Glimpse into Ionization Energy
Ionization energy, often denoted as IE, represents the minimum energy required to remove an electron from a gaseous atom in its ground electronic state, forming a positively charged ion. This fundamental property is a reflection of the strength of the electrostatic attraction between the positively charged nucleus and the negatively charged electrons.
Periodic Trends: A Dance of Opposing Forces
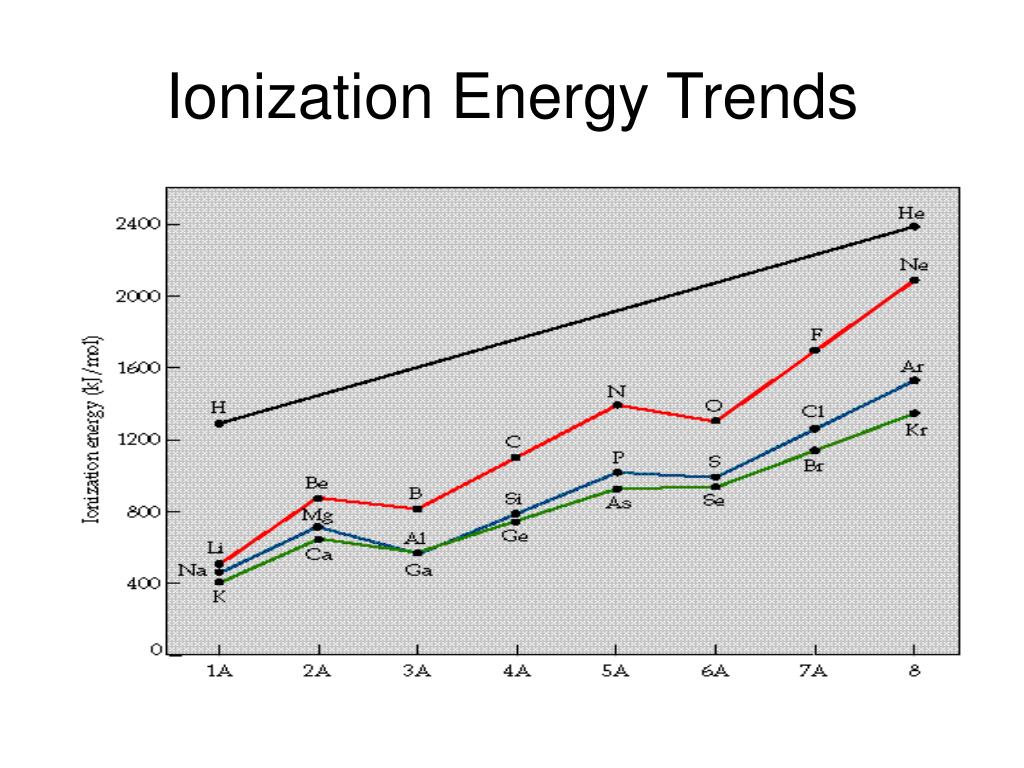
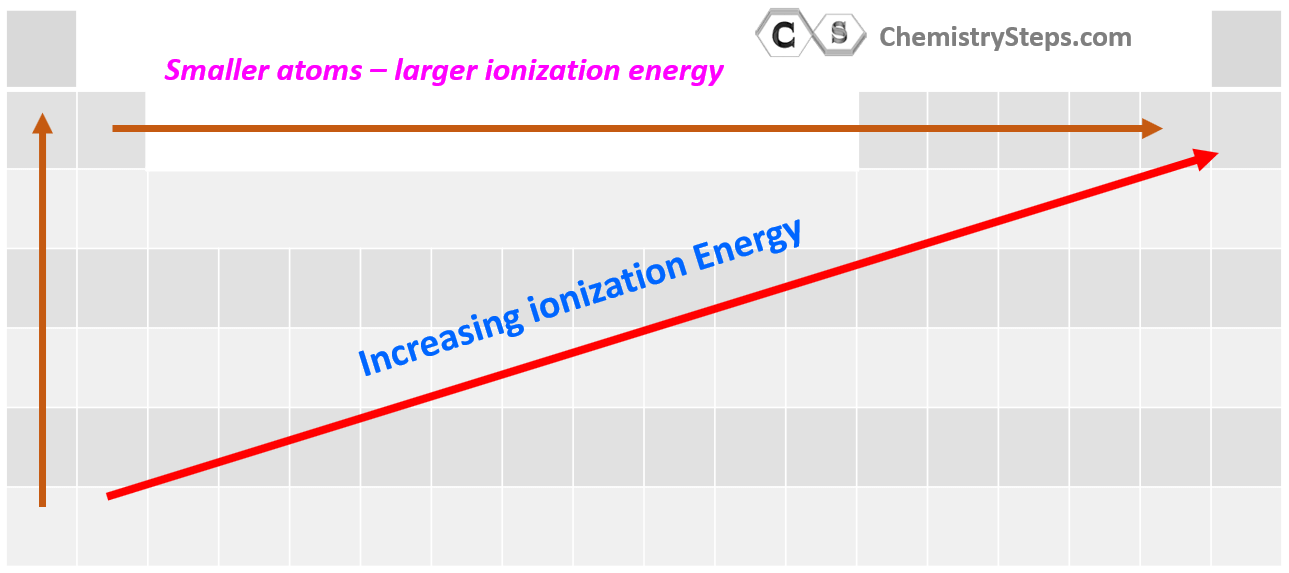
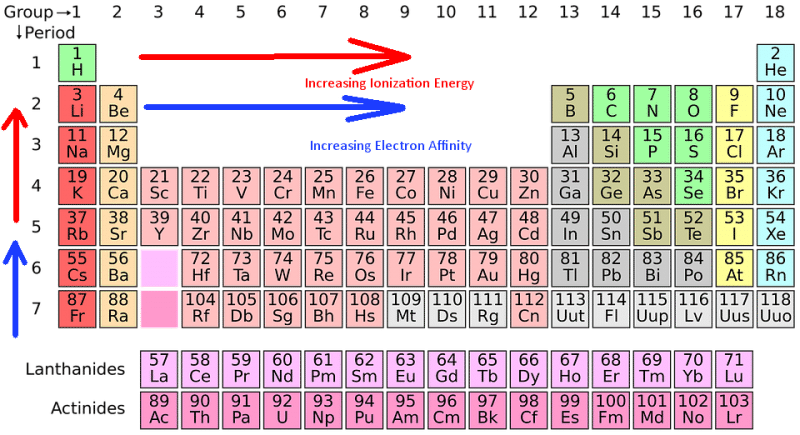
The periodic table, a testament to the order within the vast expanse of elements, serves as a powerful tool for understanding ionization energy trends. As we move across a period from left to right, ionization energy generally increases. This trend arises from the increasing nuclear charge, which exerts a stronger pull on the electrons. Conversely, as we move down a group, ionization energy generally decreases. This trend is attributed to the increasing atomic size, which weakens the attraction between the nucleus and the outermost electrons due to increased electron shielding.
However, these general trends are not absolute and are subject to subtle variations influenced by a complex interplay of factors.
The Role of Electron Configuration: Unveiling the Subatomic Dance
Electron configuration, the distribution of electrons within atomic orbitals, plays a crucial role in determining ionization energy. Electrons in different orbitals experience varying degrees of attraction to the nucleus due to differences in shielding and penetration.
- Orbital Penetration: Electrons in s orbitals, due to their spherical shape, penetrate closer to the nucleus than electrons in p orbitals. This closer proximity results in a stronger attraction to the nucleus, leading to higher ionization energies.
- Electron Shielding: Electrons in inner shells effectively shield outer electrons from the nuclear charge, reducing the attraction. This effect is more pronounced for elements with larger numbers of inner shells, leading to lower ionization energies.
These factors explain the observed anomalies in ionization energy trends. For instance, the ionization energy of nitrogen is higher than oxygen, even though oxygen has a higher nuclear charge. This anomaly is attributed to the half-filled p orbital configuration of nitrogen, which provides greater stability and requires more energy to remove an electron.
Beyond the Basics: Unveiling the Nuances
The intricacies of ionization energy extend beyond the simple trends observed in the periodic table. Several factors contribute to the subtle variations and anomalies in ionization energy values:
- Electron-Electron Repulsion: Electrons within the same shell repel each other, reducing the effective nuclear charge experienced by each electron. This effect becomes more significant with increasing electron-electron repulsion, leading to lower ionization energies.
- Relativistic Effects: For heavier elements, the electrons in the inner shells move at speeds comparable to the speed of light. This relativistic effect leads to contraction of the inner shells and a stronger attraction to the nucleus, increasing ionization energy.
- Spin-Orbit Coupling: In atoms with multiple electrons, the interaction between the electron spin and its orbital motion can lead to splitting of energy levels. This effect can influence ionization energy by affecting the energy required to remove an electron from a particular orbital.
The Frontier of Research: Unlocking the Potential of Ionization Energy
The study of ionization energy is not confined to the realm of theoretical calculations and periodic trends. Researchers are actively exploring the practical implications of ionization energy, particularly in the development of cutting-edge technologies:
- Solar Energy: Understanding ionization energy is crucial for designing efficient solar cells. The energy required to excite electrons in a material to generate electricity is directly related to its ionization energy.
- Quantum Computing: Ionization energy plays a key role in the development of quantum computers, where individual atoms are used to store and process information. The ability to manipulate ionization energy through external stimuli opens up possibilities for controlling the state of these atoms.
- Materials Science: Ionization energy is a crucial factor in determining the chemical and physical properties of materials. By tuning ionization energy, researchers can design materials with specific properties for applications ranging from catalysis to energy storage.
The Future of Ionization Energy: A Glimpse into the Uncharted Territories
The study of ionization energy continues to evolve, driven by advancements in computational techniques and experimental methods. Future research will focus on:
- Exploring the interplay between ionization energy and other atomic properties: Understanding how ionization energy relates to other fundamental properties like electron affinity, electronegativity, and atomic radius will provide a deeper understanding of chemical bonding and reactivity.
- Developing novel experimental techniques for measuring ionization energy: Advances in laser spectroscopy and photoelectron spectroscopy will enable more precise measurements of ionization energy, paving the way for a more accurate understanding of atomic and molecular interactions.
- Harnessing ionization energy for technological advancements: The potential of manipulating ionization energy through external stimuli holds immense promise for revolutionizing fields like solar energy, quantum computing, and materials science.
Conclusion: A Journey of Discovery
The study of ionization energy is a testament to the interconnectedness of the physical world. From the intricacies of atomic structure to the development of cutting-edge technologies, ionization energy continues to play a pivotal role in our understanding of matter and its properties. As we delve deeper into the fascinating world of atoms and their interactions, the insights gained from studying ionization energy will continue to shape our understanding of the universe and drive technological innovation.