Unraveling The Periodic Trends Of Electronegativity: A 2025 Perspective

Unraveling the Periodic Trends of Electronegativity: A 2025 Perspective
Electronegativity, a fundamental concept in chemistry, quantifies an atom’s ability to attract electrons within a chemical bond. This intrinsic property plays a crucial role in determining the nature of chemical bonds, the polarity of molecules, and the reactivity of elements. As we delve into the year 2025, our understanding of electronegativity has evolved significantly, fueled by advancements in computational chemistry, experimental techniques, and a deeper appreciation for the complex interplay of factors influencing this property.
Electronegativity: A Historical Journey
The concept of electronegativity was first introduced by Linus Pauling in 1932, who recognized its significance in explaining the behavior of chemical bonds. Pauling’s scale, based on bond energies, remains widely used today. However, other scales have emerged, such as the Mulliken scale, which relates electronegativity to ionization energy and electron affinity. These scales, while differing in their specific definitions, all capture the essence of electronegativity – an atom’s relative tendency to attract electrons.
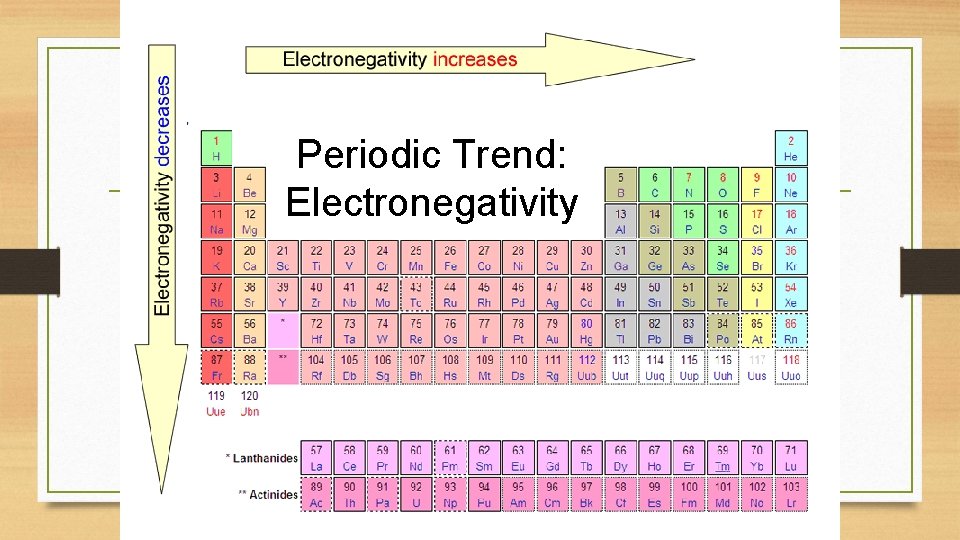
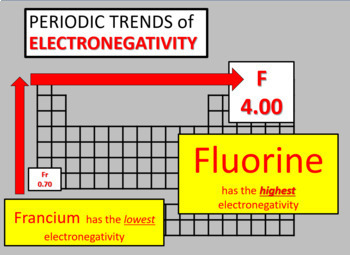
Periodic Trends: Unveiling the Patterns
Electronegativity exhibits clear periodic trends, reflecting the underlying electronic structure of elements. As we move across a period from left to right, the electronegativity generally increases. This is attributed to the increasing nuclear charge, which attracts valence electrons more strongly. As we move down a group, electronegativity generally decreases due to the increasing atomic size, shielding the valence electrons from the nucleus.
Beyond the Basics: Refining the Understanding
While the periodic trends provide a general framework, understanding electronegativity requires a nuanced approach. Several factors contribute to the variations observed:
- Effective Nuclear Charge: While nuclear charge increases across a period, the shielding effect of inner electrons also plays a role. The effective nuclear charge, the net positive charge experienced by valence electrons, is a crucial factor in determining electronegativity.
- Electron Configuration: The specific electronic configuration of an element, particularly the number of valence electrons, influences its electronegativity. Elements with half-filled or completely filled valence shells tend to be more electronegative.
- Atomic Size: As mentioned earlier, atomic size plays a significant role. Larger atoms, with their valence electrons farther from the nucleus, experience weaker attraction and exhibit lower electronegativity.
- Hybridization: The hybridization of atomic orbitals, particularly in covalent bonding, can influence the electronegativity of an atom. Hybridization can lead to a more localized electron distribution, impacting the attraction towards bonding electrons.
- Relativistic Effects: For heavier elements, relativistic effects become increasingly important. These effects, arising from the high speeds of electrons near the nucleus, can alter the electronic configuration and influence electronegativity.
The Role of Computational Chemistry
Computational chemistry has revolutionized our ability to study electronegativity. Quantum chemical calculations, based on the principles of quantum mechanics, allow us to calculate accurate electronegativity values for various elements and even predict how electronegativity changes in different chemical environments. These methods provide valuable insights into the underlying electronic structure and bonding characteristics.
Electronegativity in Action: Applications and Implications
Electronegativity is not merely a theoretical concept; it has profound practical implications across diverse fields:
- Predicting Bond Polarity: The difference in electronegativity between two atoms participating in a bond determines its polarity. A large electronegativity difference results in a polar bond, where electrons are unevenly distributed, creating partial charges. This polarity is crucial for understanding the behavior of molecules, such as water, which exhibits strong dipole-dipole interactions due to the polar O-H bonds.
- Understanding Chemical Reactivity: Electronegativity plays a vital role in predicting the reactivity of elements and compounds. Highly electronegative atoms tend to attract electrons from other atoms, leading to the formation of anions. Conversely, elements with low electronegativity readily lose electrons, forming cations. This understanding is crucial for designing chemical reactions and predicting their outcomes.
- Developing New Materials: The ability to control and manipulate electronegativity is essential for designing materials with specific properties. For example, by tuning the electronegativity of constituent atoms, researchers can create materials with desired electrical conductivity, optical properties, or catalytic activity.
- Understanding Biological Systems: Electronegativity plays a crucial role in understanding the behavior of biological molecules. The polar nature of many biomolecules, such as proteins and nucleic acids, arises from the presence of polar bonds due to electronegativity differences. This polarity influences the interactions between molecules, contributing to the complex functions of biological systems.
Future Directions: Expanding the Horizons
As we move forward, the study of electronegativity continues to evolve:
- Advanced Computational Methods: The development of more sophisticated computational methods, incorporating relativistic effects and complex electron correlation, will enable more accurate and reliable predictions of electronegativity values.
- Exploring New Scales: The search for new electronegativity scales, tailored to specific applications and taking into account additional factors, will continue to refine our understanding of this fundamental property.
- Understanding the Dynamic Nature of Electronegativity: Electronegativity is not a static property but can vary depending on the chemical environment and the specific bonding situation. Future research will focus on understanding how electronegativity evolves dynamically within molecules and how this variation impacts chemical reactivity and material properties.
- Developing Novel Applications: The understanding of electronegativity will continue to drive advancements in various fields, including materials science, catalysis, and drug design. By manipulating electronegativity, researchers will strive to create materials with tailored properties and develop new technologies with enhanced functionalities.
Conclusion: A Foundation for Chemical Understanding
Electronegativity remains a cornerstone of chemical understanding, providing a framework for explaining and predicting the behavior of atoms and molecules. As we enter the year 2025, our knowledge of electronegativity has deepened significantly, thanks to the integration of computational chemistry, experimental techniques, and a nuanced appreciation for the factors influencing this property. The future holds exciting prospects for further unraveling the complexities of electronegativity and leveraging this understanding to advance scientific discovery and technological innovation.
.PNG)



/chart-of-periodic-table-trends-608792-v1-6ee35b80170349e8ab67865a2fdfaceb.png)