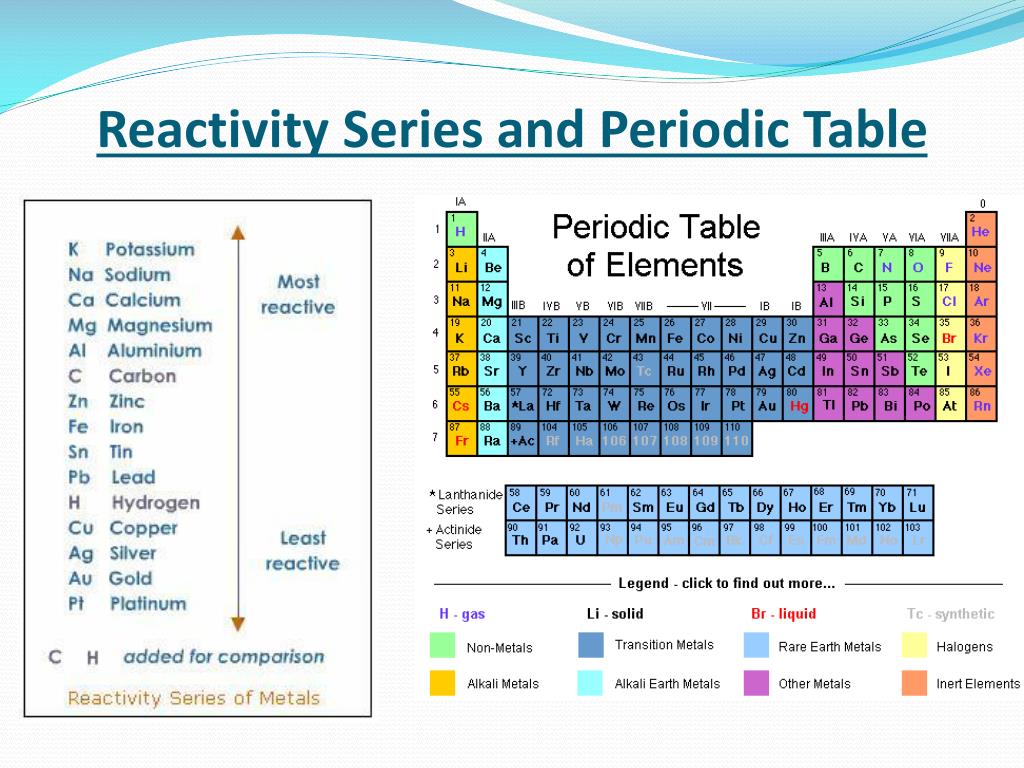
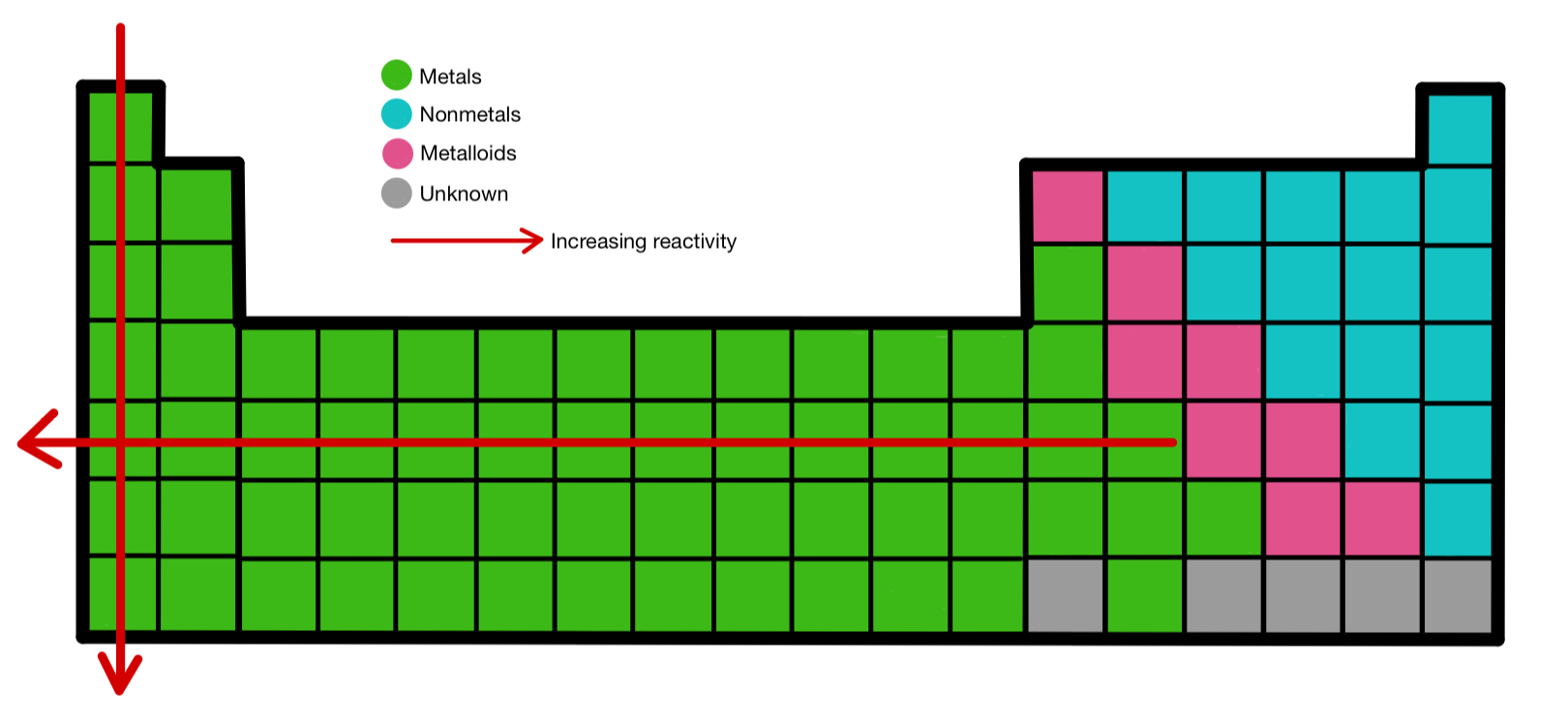
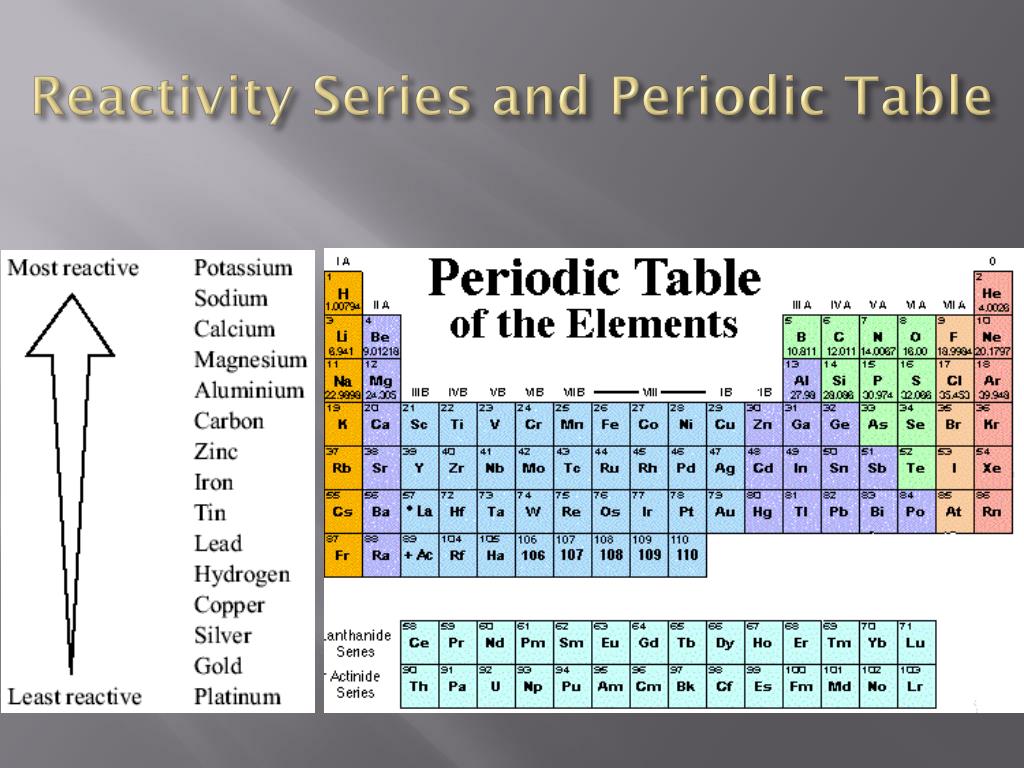
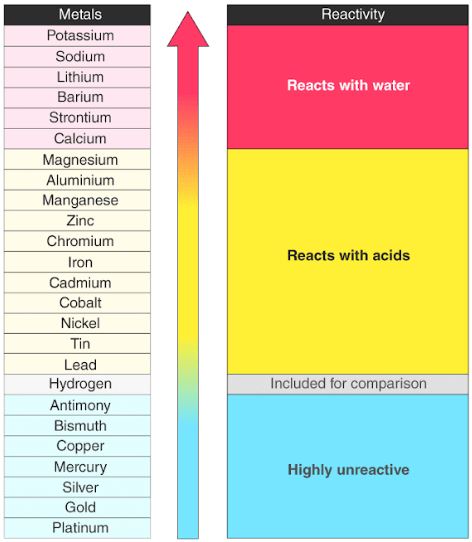
The Periodic Table In 2025: Unveiling New Trends In Reactivity
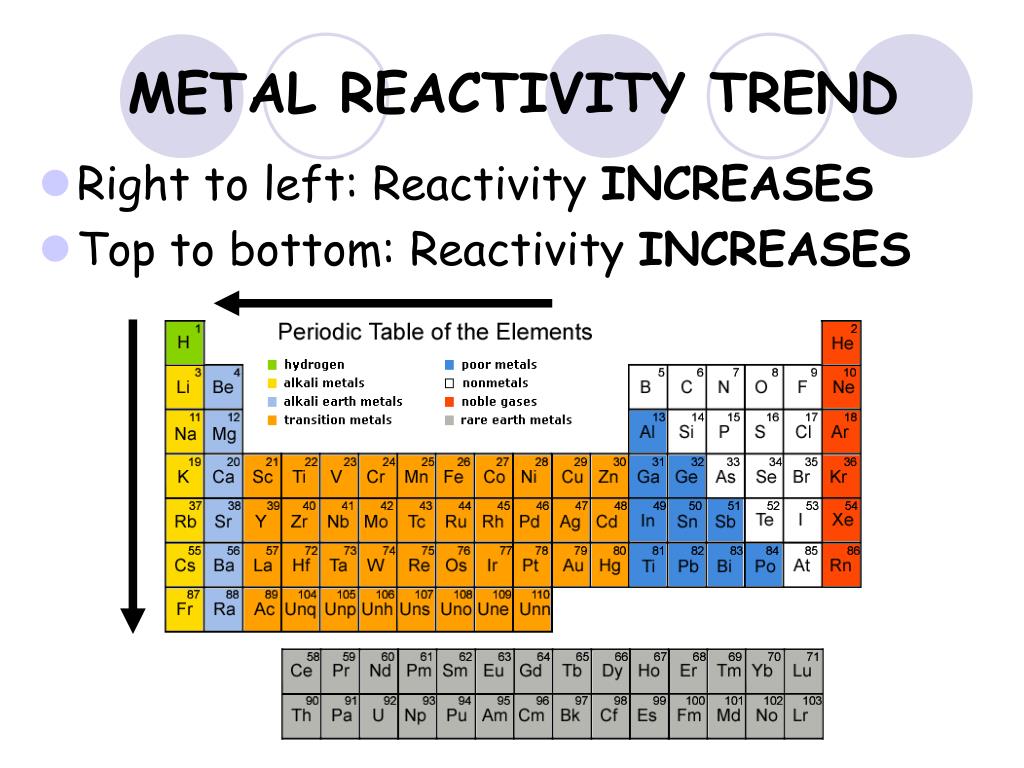
The Periodic Table in 2025: Unveiling New Trends in Reactivity
The periodic table, a cornerstone of chemistry, continues to evolve, revealing new insights into the fascinating world of chemical reactivity. As we stand at the cusp of 2025, advancements in computational chemistry, experimental techniques, and the discovery of novel elements have yielded a deeper understanding of atomic behavior and its influence on chemical reactions. This article delves into the latest trends in periodic table reactivity, exploring how these discoveries are shaping the future of chemistry, materials science, and beyond.
Beyond the Basics: Redefining Reactivity Trends
The traditional periodic table trends, such as electronegativity, ionization energy, and electron affinity, provide a fundamental framework for understanding reactivity. However, these trends often fail to capture the nuances of complex chemical systems. In 2025, the focus has shifted towards a more nuanced understanding of reactivity, incorporating factors like:
-
Relativistic Effects: The influence of relativistic effects, particularly for heavier elements, is increasingly recognized. These effects, arising from the high speeds of electrons in heavy atoms, can significantly alter electron configurations and reactivity patterns. For instance, gold’s unusual inertness is attributed to relativistic effects, which stabilize its 6s electrons, making them less prone to participating in chemical reactions.
-
Ligand Field Theory: This theory, which focuses on the interaction of metal ions with ligands, plays a crucial role in predicting and explaining the reactivity of transition metal complexes. By understanding how ligands influence the electronic structure of metal ions, we can tailor the reactivity of these complexes for specific applications.
-
Computational Modeling: Advances in computational chemistry have enabled the development of sophisticated models that can predict and simulate chemical reactions with unprecedented accuracy. These models can be used to study complex reaction pathways, identify potential reaction intermediates, and even predict the reactivity of novel compounds before they are synthesized.
Unveiling the Secrets of the Main Group Elements
The main group elements, encompassing groups 1-18, are often perceived as predictable in their reactivity. However, recent research has challenged this notion, revealing surprising and intriguing reactivity patterns.
-
The Rise of the Pnictogens: The pnictogens (group 15 elements) are gaining recognition for their diverse and often unexpected reactivity. For example, phosphorus, traditionally known for its role in fertilizers, is now being explored for its potential in energy storage applications. Its ability to form stable polyphosphides, with varying oxidation states, has opened doors for the development of high-capacity batteries.
-
The Versatility of Chalcogens: The chalcogens (group 16 elements) are known for their diverse bonding capabilities, leading to a wide range of compounds with unique properties. Recent research has focused on harnessing these properties for applications in catalysis, materials science, and even medicine. For example, selenium, traditionally associated with its role in human health, is now being investigated for its potential in developing novel solar cells and thermoelectric materials.
Navigating the Complex World of Transition Metals
Transition metals, found in groups 3-12, are renowned for their complex and often unpredictable reactivity. Their ability to form a wide range of oxidation states, coupled with their d-orbital electrons, leads to a plethora of possible reaction pathways.
-
Catalysis: The Heart of Chemical Transformations: Transition metals are the backbone of many catalytic processes, driving essential reactions in industries ranging from pharmaceuticals to plastics. In 2025, research is focused on developing new and efficient catalysts based on transition metals, particularly those that are abundant, sustainable, and environmentally friendly.
-
Beyond Catalysis: Exploring New Frontiers: The unique properties of transition metals are also being explored in fields beyond catalysis. For example, their ability to absorb and emit light is leading to the development of novel light-emitting diodes (LEDs) and solar cells. Their magnetic properties are also being utilized in the development of next-generation magnetic storage devices and sensors.
Uncharted Territories: The Future of Superheavy Elements
The periodic table is not static; it continues to expand with the discovery of new elements. The synthesis and characterization of superheavy elements, those beyond uranium, are pushing the boundaries of our understanding of chemical reactivity.
-
Relativistic Effects Dominate: The extreme relativistic effects experienced by superheavy elements significantly alter their electronic structure and reactivity. These effects are so pronounced that they can even lead to the formation of unexpected chemical bonds and the creation of novel compounds.
-
Predicting the Unpredictable: The study of superheavy elements presents a unique challenge. Due to their short half-lives, only a few atoms can be synthesized at a time, making experimental characterization extremely difficult. However, computational models are proving invaluable in predicting the properties and reactivity of these elusive elements, paving the way for future discoveries.
The Impact of Periodic Table Trends on Our Future
The evolving understanding of periodic table trends has profound implications for various fields:
-
Materials Science: By understanding the reactivity of different elements, scientists can design new materials with tailored properties for specific applications. This includes developing stronger, lighter, and more durable materials for construction, aerospace, and electronics.
-
Medicine: The development of new drugs and therapies often hinges on understanding the reactivity of specific elements. For example, the use of platinum-based drugs in cancer treatment is a testament to the importance of understanding the reactivity of transition metals.
-
Environmental Sustainability: The search for more sustainable and environmentally friendly chemical processes relies on understanding the reactivity of elements. This includes developing new catalysts for cleaner energy production and reducing the environmental impact of industrial processes.
Conclusion: A Never-Ending Journey of Discovery
The periodic table is not just a static chart; it’s a dynamic roadmap guiding us through the intricate world of chemical reactivity. As we continue to explore the periodic table’s depths, we unlock new possibilities for innovation and advancement in fields ranging from materials science to medicine. The discoveries of 2025 and beyond will undoubtedly rewrite our understanding of chemical reactivity, paving the way for a future where the periodic table serves as a springboard for transformative breakthroughs.
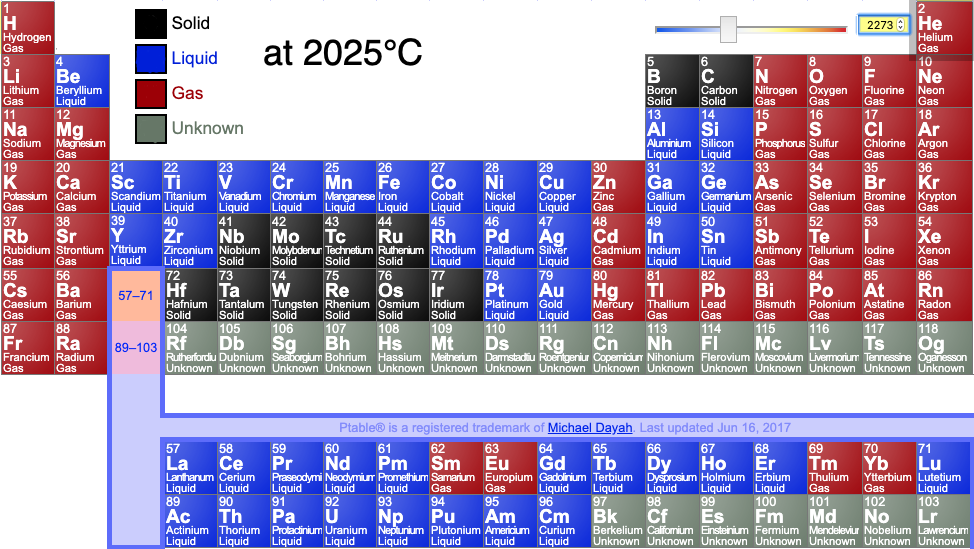
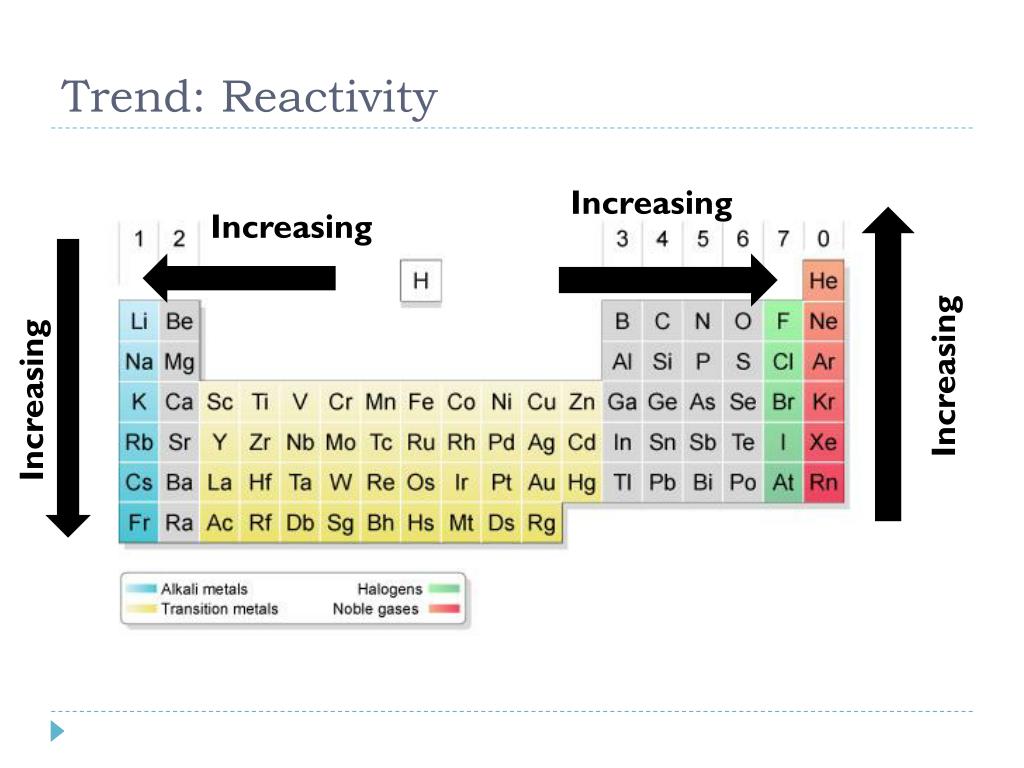
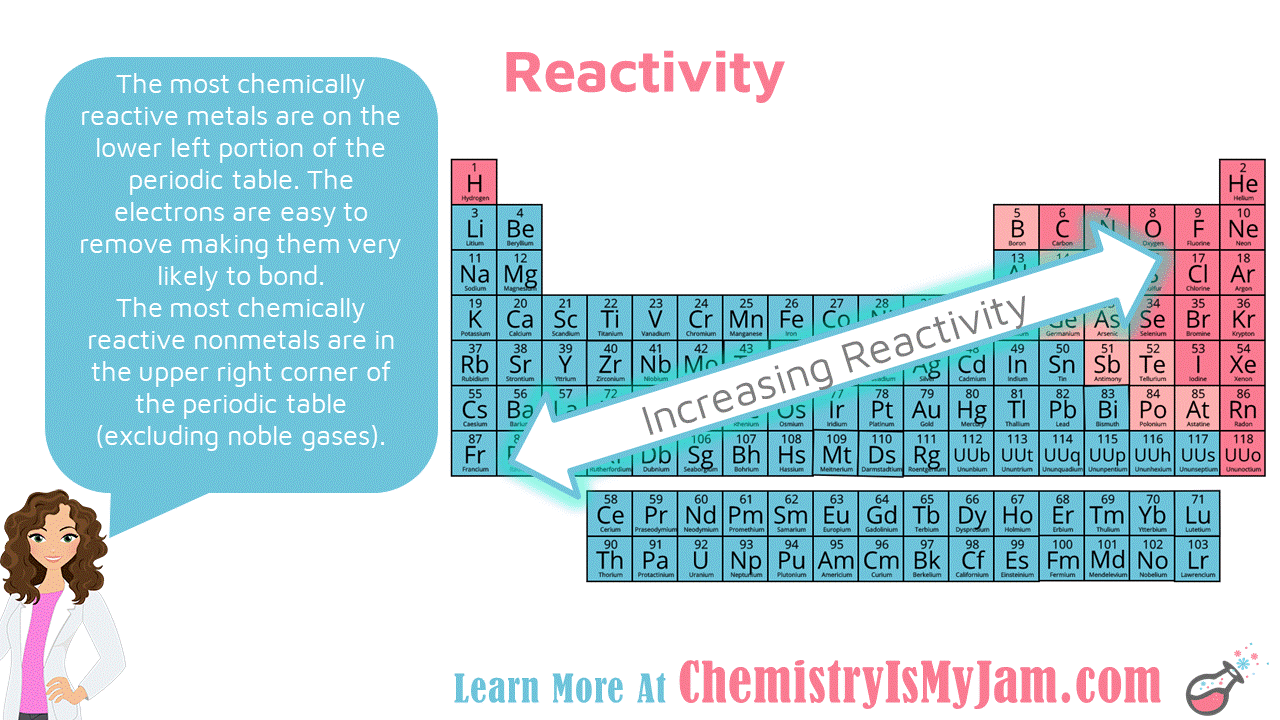
:max_bytes(150000):strip_icc()/chart-of-periodic-table-trends-608792-v1-6ee35b80170349e8ab67865a2fdfaceb.png)