The Periodic Dance Of Electrons: Unveiling The Trends In Ionization Energy In 2025
The Periodic Dance of Electrons: Unveiling the Trends in Ionization Energy in 2025
The periodic table, a cornerstone of chemistry, is not merely a static chart of elements but a dynamic map reflecting the intricate dance of electrons. At its core lies the concept of ionization energy, a fundamental property that dictates how readily an atom sheds an electron, revealing the secrets of chemical reactivity and bonding. As we stand on the cusp of 2025, our understanding of this crucial property continues to evolve, driven by advancements in theoretical calculations and experimental techniques. This article delves into the fascinating trends of ionization energy across the periodic table, exploring the underlying principles and their implications for our understanding of the chemical world.
A Glimpse into the Realm of Electrons:
Ionization energy (IE) is the minimum energy required to remove an electron from a gaseous atom in its ground electronic state, forming a positively charged ion. This energy, measured in electron volts (eV), is a direct reflection of the strength of the electrostatic attraction between the positively charged nucleus and the negatively charged electron. The higher the ionization energy, the more tightly bound the electron is to the atom, and the less readily it will be removed.
Trends Across the Periodic Table:
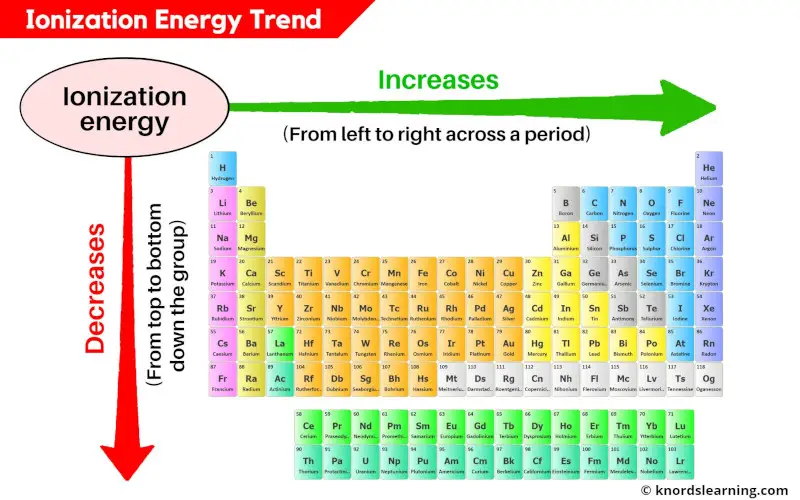
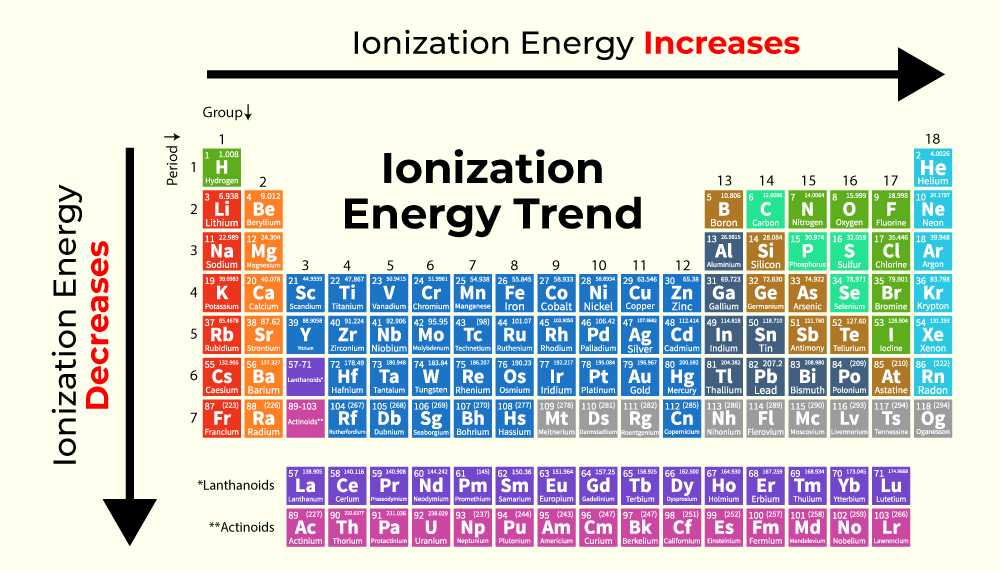
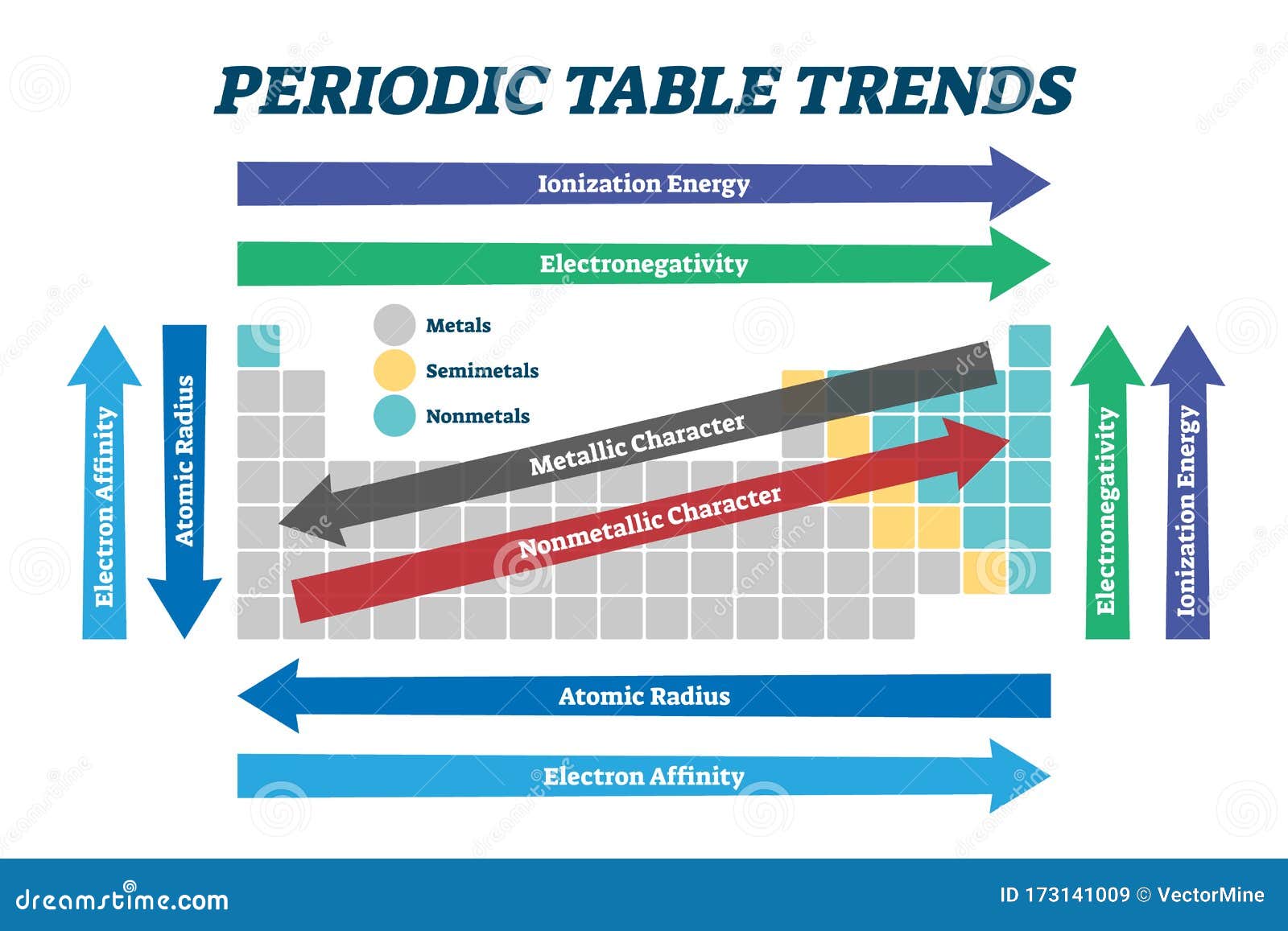
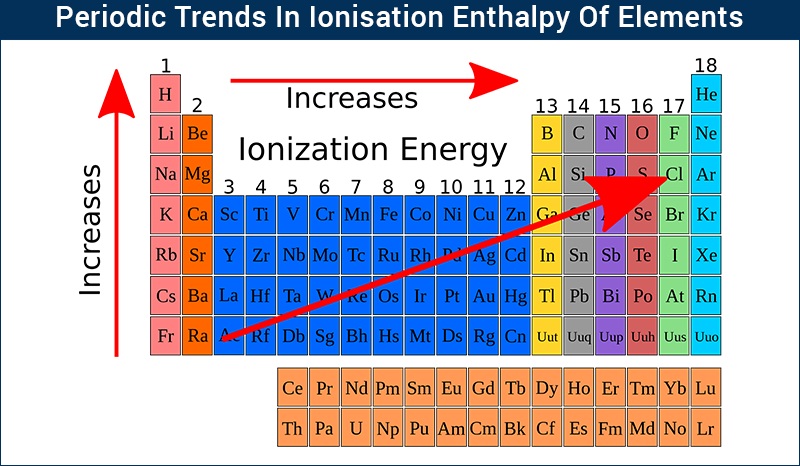
The periodic table serves as a visual guide to the trends in ionization energy, revealing a systematic pattern of increasing ionization energy as we move:
-
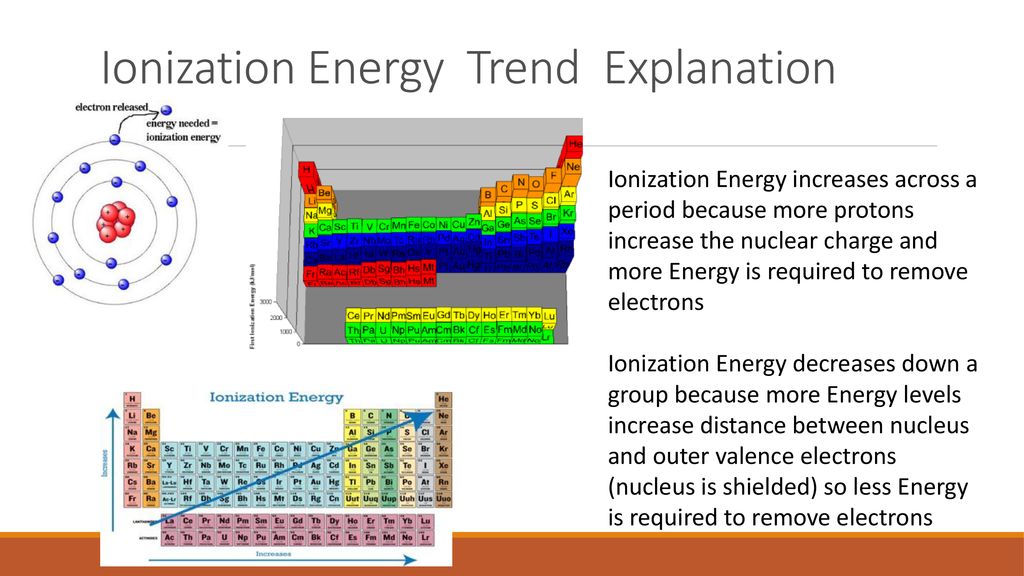
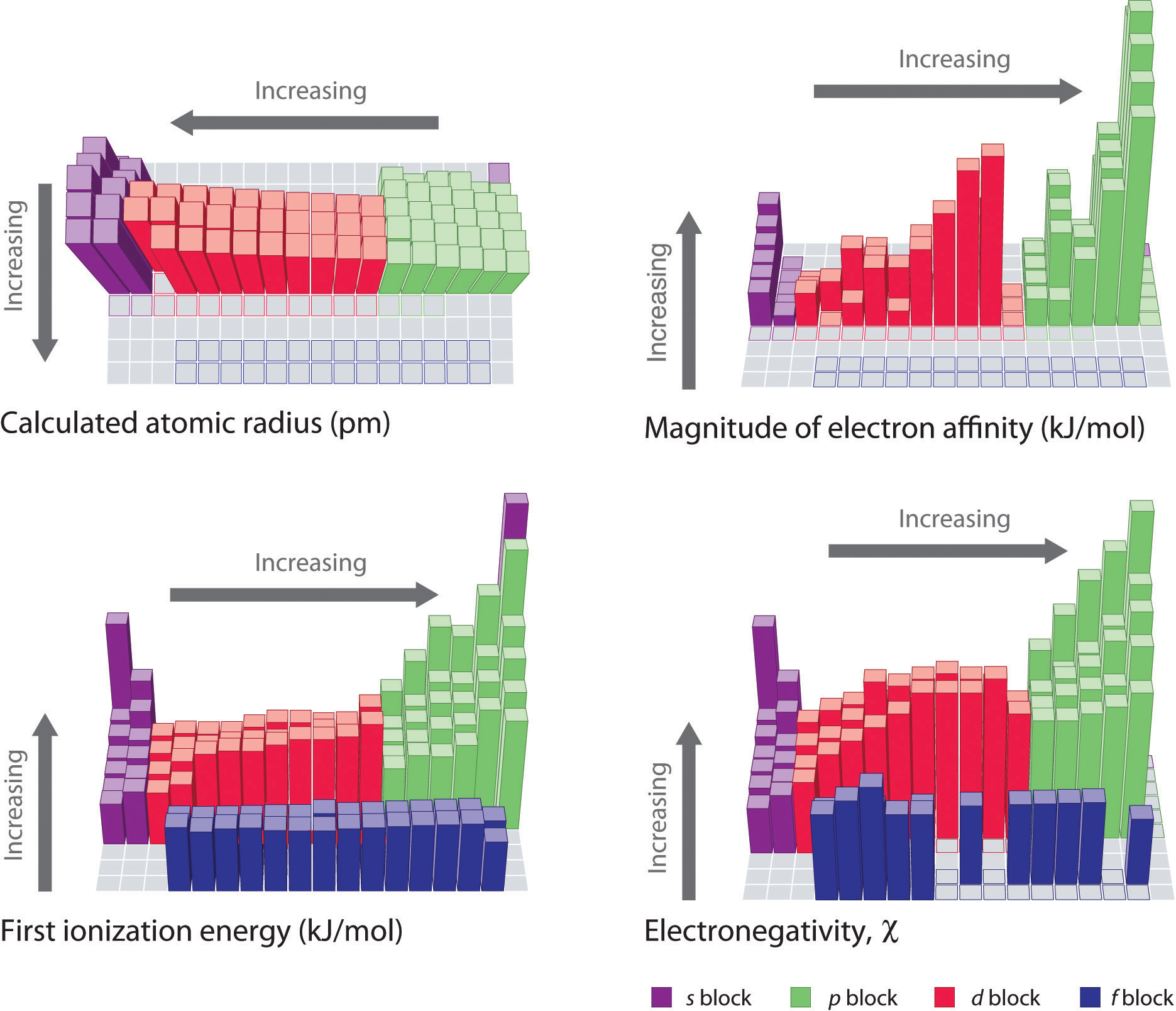
Across a period (from left to right): Ionization energy generally increases. This is because the number of protons in the nucleus increases, leading to a stronger attraction for the electrons. Furthermore, the electrons are added to the same energy level, with only a slight increase in shielding effect. Consequently, the electrons are held more tightly, resulting in higher ionization energies.
-
Down a group (from top to bottom): Ionization energy generally decreases. As we move down a group, the principal quantum number (n) increases, meaning the electrons are located further from the nucleus. This increased distance weakens the electrostatic attraction, making it easier to remove an electron, thus lowering the ionization energy. Additionally, the increasing number of electron shells leads to a greater shielding effect, further reducing the attraction between the nucleus and the valence electrons.
Beyond the Basics: Unveiling the Nuances:
While these general trends provide a solid framework, understanding the nuances within the periodic table requires a deeper dive. Factors like electron configuration, shielding effects, and the presence of filled or half-filled subshells can significantly impact ionization energy.
Electron Configuration and Ionization Energy:
The electron configuration of an atom plays a crucial role in determining its ionization energy. Elements with a stable electron configuration, like those with a completely filled or half-filled subshell, tend to have higher ionization energies. For example, nitrogen (N) with a half-filled p subshell (2p3) exhibits a higher ionization energy compared to its neighbor, oxygen (O), due to the extra stability associated with the half-filled subshell.
Shielding Effects and Ionization Energy:
Shielding refers to the effect of inner electrons in reducing the attraction between the nucleus and the valence electrons. The greater the shielding, the weaker the attraction, and the lower the ionization energy. For example, the ionization energy of sodium (Na) is significantly lower than that of lithium (Li) due to the presence of additional inner electrons in sodium, which shield the valence electron from the nucleus.
Exceptions to the Trends:
While the general trends of ionization energy hold true for most elements, some exceptions exist. For instance, the ionization energy of beryllium (Be) is higher than that of boron (B), despite being located to the left of boron in the periodic table. This anomaly arises from the fact that beryllium has a filled 2s subshell, which contributes to its greater stability and higher ionization energy.
The Role of Ionization Energy in Chemical Reactivity:
Ionization energy is a crucial parameter in understanding the chemical reactivity of elements. Elements with low ionization energies tend to readily lose electrons, making them good reducing agents. Conversely, elements with high ionization energies tend to hold onto their electrons tightly, making them poor reducing agents.
Applications and Impact:
The study of ionization energy has profound implications in various fields:
-
Predicting Chemical Reactions: Understanding ionization energies allows us to predict the likelihood of chemical reactions and the formation of ionic bonds.
-
Designing Materials: By controlling the ionization energy of materials, scientists can tailor their properties for specific applications, such as developing new catalysts, semiconductors, and solar cells.
-
Astrophysical Studies: Ionization energies play a crucial role in understanding the composition and evolution of stars and interstellar clouds.
Future Perspectives:
As we move into 2025 and beyond, the field of ionization energy research continues to evolve. Advancements in computational chemistry allow for more accurate predictions of ionization energies, while new experimental techniques provide deeper insights into the dynamics of electron removal.
Conclusion:
Ionization energy is a fundamental property that governs the chemical behavior of elements. The trends observed across the periodic table provide a powerful framework for understanding and predicting chemical reactivity. As our understanding of this property continues to deepen, we can expect even more exciting breakthroughs in various scientific disciplines, from materials science to astrophysics. The periodic dance of electrons, as revealed by ionization energy, continues to captivate and inspire scientists, unlocking the secrets of the chemical world and shaping our future.

.PNG)
:max_bytes(150000):strip_icc()/PeriodicTable-Trends-56a1310e5f9b58b7d0bcea8a.png)