The Dance Of Electrons: Exploring Atomic Radius Trends In 2025

The Dance of Electrons: Exploring Atomic Radius Trends in 2025
The periodic table, a cornerstone of chemistry, is a map of the elements, their properties, and their interconnectedness. One fundamental property that governs the behavior of atoms and molecules is atomic radius, the distance from the nucleus to the outermost electron shell. Understanding atomic radius trends is crucial for predicting chemical reactivity, bonding characteristics, and the formation of various materials.
In 2025, our understanding of atomic radius has evolved significantly, driven by advancements in experimental techniques, theoretical models, and the development of new materials. This article delves into the current state of knowledge regarding atomic radius trends, exploring both the established principles and the frontiers of research.
A Tale of Two Forces:
The dance of electrons within an atom is a delicate balance between two opposing forces: the attraction of the positively charged nucleus and the repulsion between negatively charged electrons. This interplay dictates the size of an atom, with two key factors influencing atomic radius:
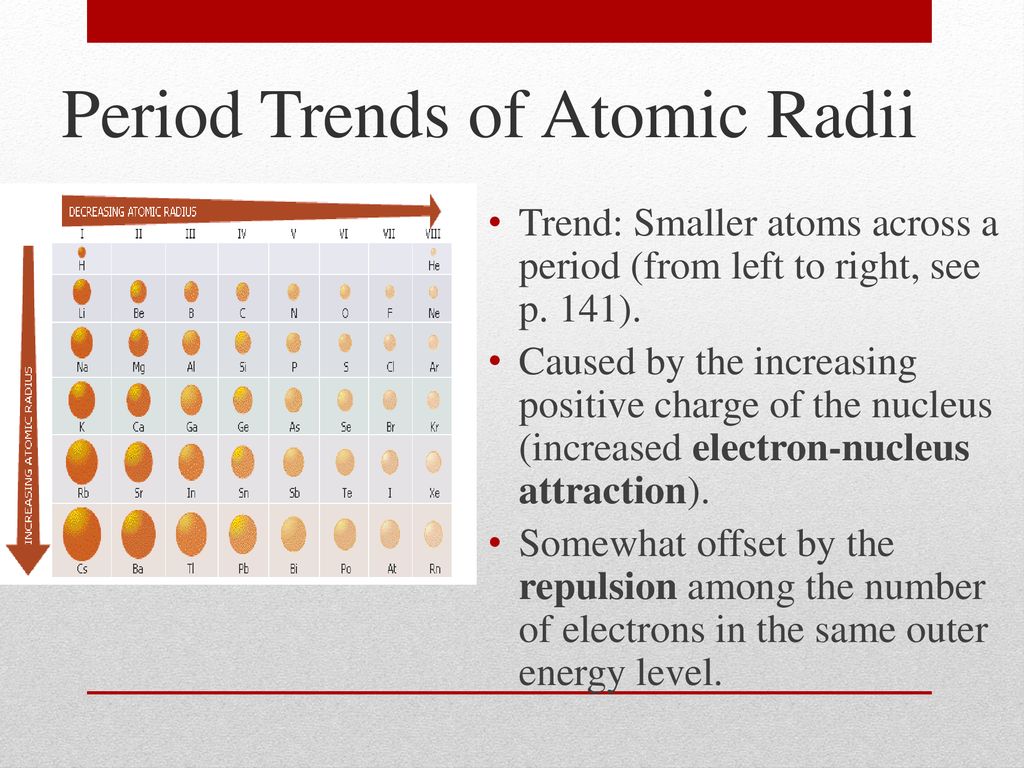
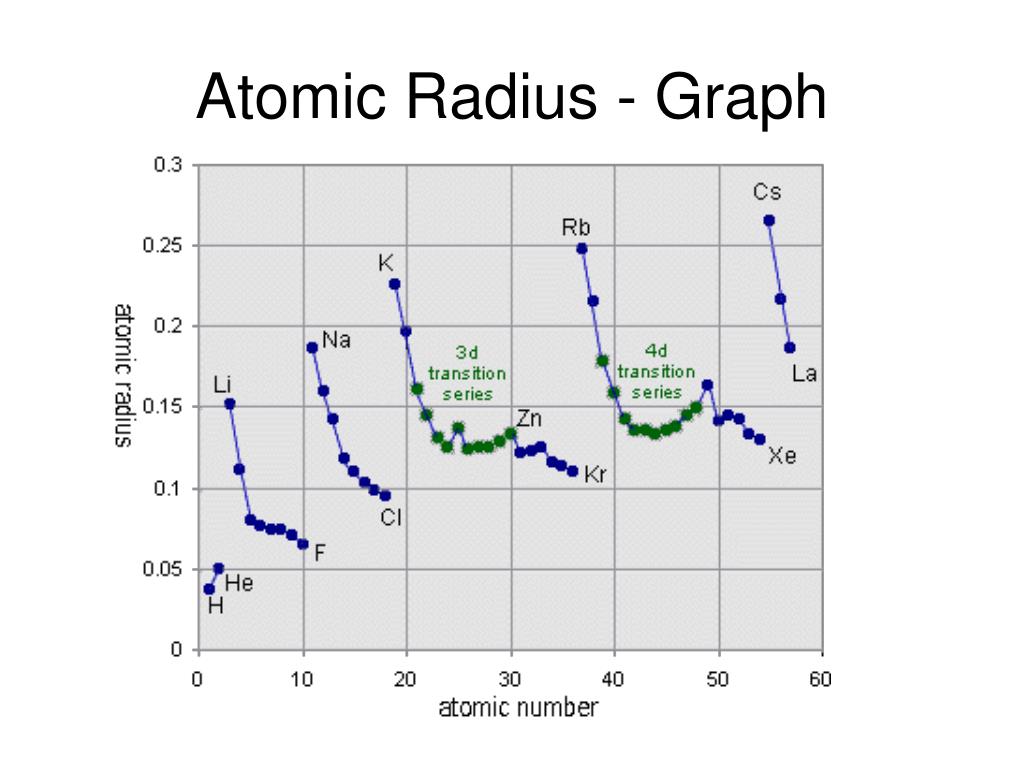
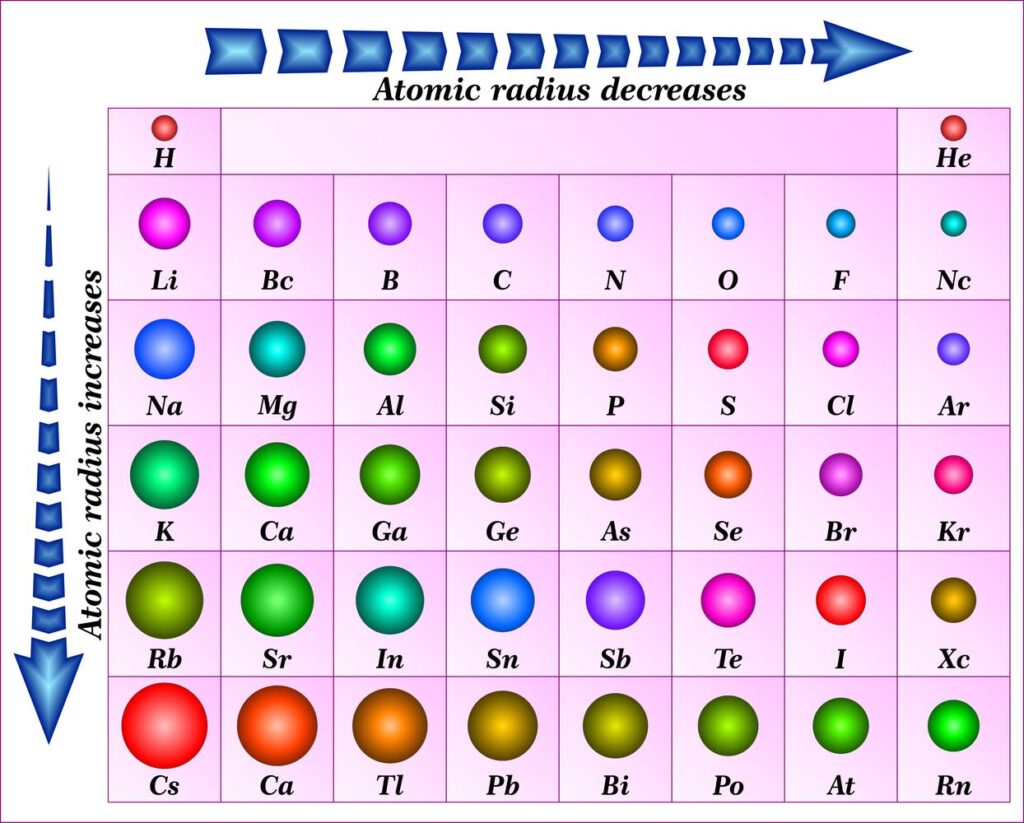
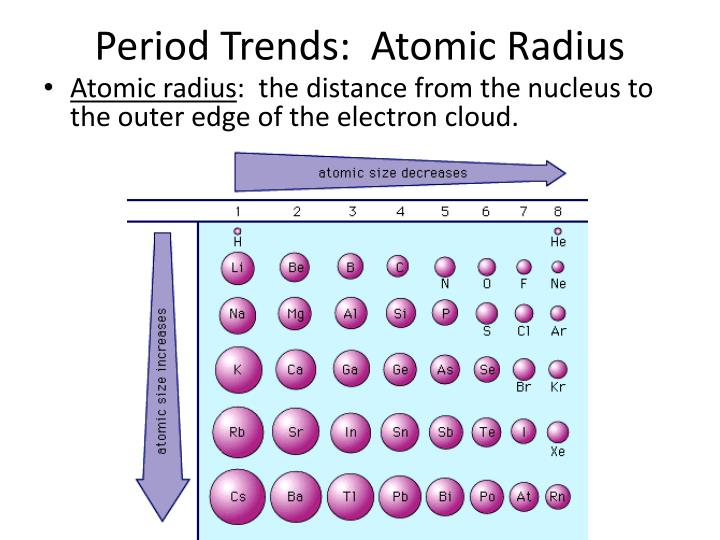
- Nuclear Charge (Z): As the number of protons in the nucleus (Z) increases, the electrostatic attraction between the nucleus and electrons strengthens, pulling them closer and shrinking the atomic radius. This trend is evident when moving across a period from left to right.
- Electron Shielding: Electrons in inner shells shield outer electrons from the full pull of the nucleus. This shielding effect reduces the effective nuclear charge experienced by the outer electrons, allowing them to spread out further, increasing the atomic radius. This trend is observed when moving down a group in the periodic table.
The Periodic Dance:
These fundamental forces create a predictable pattern of atomic radius trends across the periodic table:
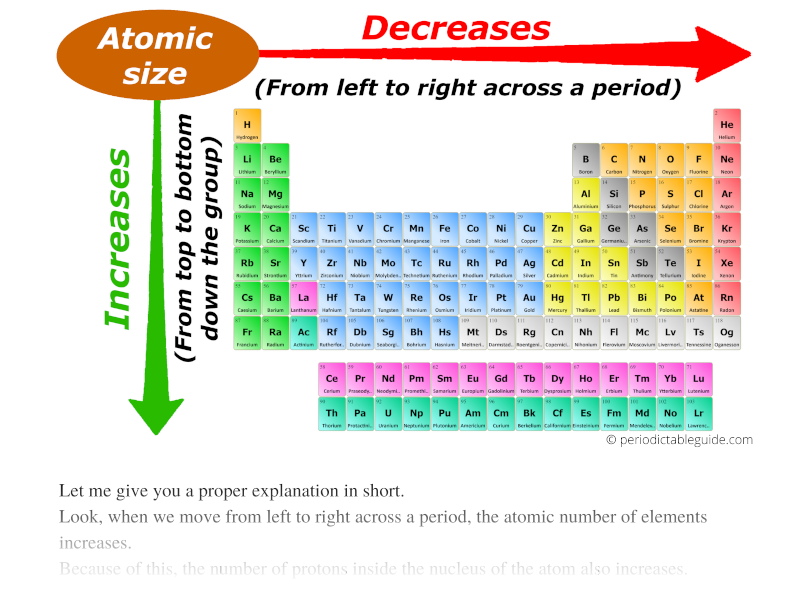
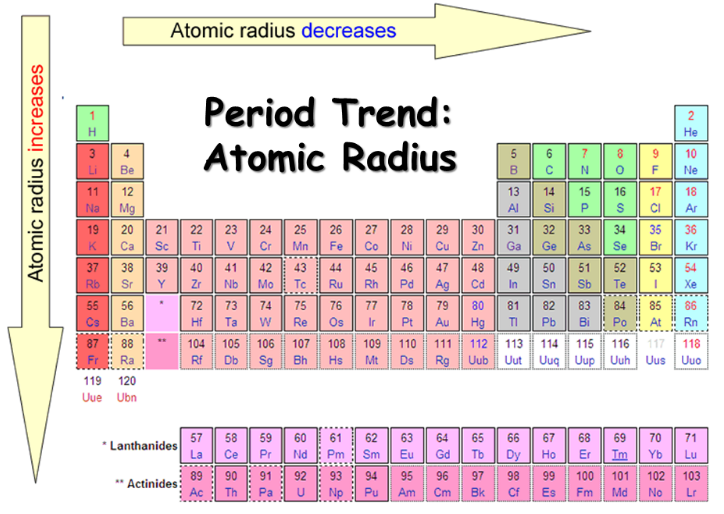
- Across a Period: As we move from left to right across a period, the number of protons in the nucleus increases, leading to a stronger attraction between the nucleus and electrons. Despite adding electrons to the same shell, the increased nuclear charge dominates, resulting in a decrease in atomic radius.
- Down a Group: As we move down a group, the number of electron shells increases. The addition of new shells effectively shields outer electrons from the nucleus, reducing the effective nuclear charge. This weaker attraction allows the outer electrons to spread out further, leading to an increase in atomic radius.
Beyond the Basics: Refinements and Nuances
While these general trends provide a valuable framework, the reality of atomic radius is more nuanced. Several factors contribute to deviations from the idealized picture:
- Electron Configuration: The specific electron configuration of an element influences its atomic radius. Atoms with half-filled or fully filled subshells exhibit a slight increase in atomic radius due to enhanced stability.
- Relativistic Effects: For heavier elements, the velocity of electrons approaches a significant fraction of the speed of light. Relativistic effects, such as the increase in electron mass, influence the size of the atom, leading to a contraction of the atomic radius.
- Ionic Radius: The formation of ions, whether cations (positively charged) or anions (negatively charged), significantly alters the atomic radius. Cations are smaller than their parent atoms due to the loss of electrons, while anions are larger due to the gain of electrons.
The Atomic Radius Landscape in 2025:
In 2025, our understanding of atomic radius has advanced beyond the traditional periodic trends. New techniques and theoretical models have enabled a more precise and comprehensive view:
- Quantum Chemical Calculations: Advanced computational methods, such as density functional theory (DFT), provide highly accurate predictions of atomic radii, taking into account electron correlation and relativistic effects.
- X-ray Diffraction: This technique allows us to determine the precise atomic arrangement in crystalline materials, providing experimental data on atomic radii.
- Atomic Force Microscopy (AFM): AFM allows us to visualize and measure the surface topography of materials at the atomic level, providing insights into atomic radii and surface interactions.
The Importance of Atomic Radius:
Understanding atomic radius trends is crucial for various fields, including:
- Chemistry: Atomic radius influences the bond lengths, bond angles, and reactivity of molecules. It also plays a role in predicting the stability of chemical compounds and their properties.
- Materials Science: The size of atoms influences the packing density of materials, their mechanical properties, and their electronic conductivity. Understanding atomic radius is essential for designing new materials with specific properties.
- Nanotechnology: At the nanoscale, the size of atoms becomes paramount. The ability to control and manipulate atoms at this level requires a deep understanding of atomic radius and its influence on nanoscale structures and properties.
Future Directions:
Research on atomic radius continues to evolve, driven by the need to understand complex phenomena and design new materials with tailored properties:
- Exploring Atomic Radius in Exotic Materials: The study of atomic radius in exotic materials, such as superconductors, topological insulators, and two-dimensional materials, is crucial for understanding their unique properties and developing novel technologies.
- Developing New Theoretical Models: The development of more sophisticated theoretical models, incorporating relativistic effects and electron correlation, will improve the accuracy of atomic radius predictions.
- Bridging the Gap between Theory and Experiment: Combining experimental techniques like X-ray diffraction and AFM with theoretical calculations will provide a more complete picture of atomic radius and its influence on material properties.
Conclusion:
Atomic radius, a fundamental property of atoms, plays a pivotal role in shaping the world around us. From the chemical bonds that hold molecules together to the structure of materials that define our technology, the size of atoms dictates their behavior. In 2025, our understanding of atomic radius continues to evolve, driven by advancements in experimental techniques, theoretical models, and the exploration of new materials. As we delve deeper into the dance of electrons, we unlock the secrets of the atomic world and pave the way for future innovations.
/chart-of-periodic-table-trends-608792-v1-6ee35b80170349e8ab67865a2fdfaceb.png)