The Atomic Radius: A Journey Through The Periodic Table In 2025

The Atomic Radius: A Journey Through the Periodic Table in 2025
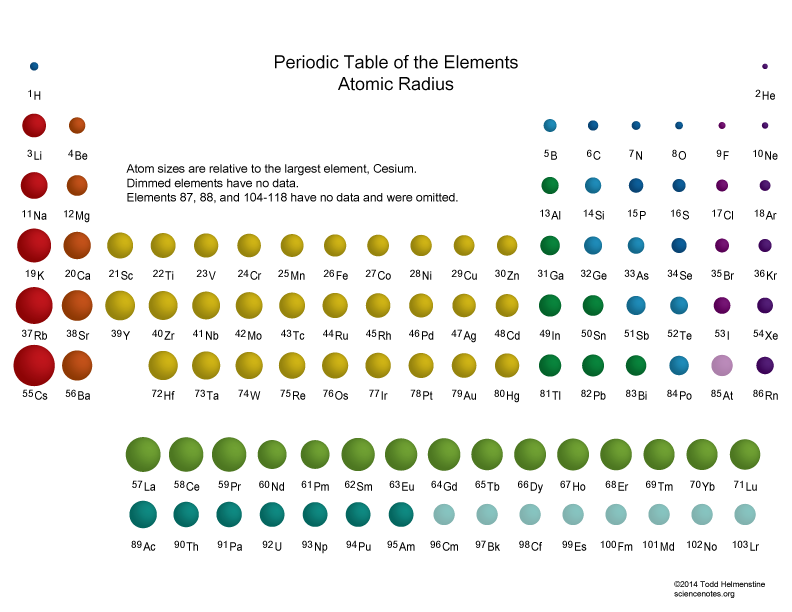
The periodic table, a cornerstone of chemistry, organizes elements based on their recurring properties. Among these properties, atomic radius stands out as a fundamental parameter, influencing a vast array of chemical and physical phenomena. In 2025, our understanding of atomic radius has advanced significantly, driven by cutting-edge experimental techniques and sophisticated theoretical models. This article explores the fascinating trends in atomic radius across the periodic table, shedding light on the interplay between electronic structure, nuclear charge, and interatomic forces.
Defining the Atomic Radius: A Challenge of Measurement
The concept of atomic radius seems straightforward: it represents the distance from the nucleus to the outermost electron shell of an atom. However, the reality is more complex. Atoms do not have well-defined boundaries, as electrons exist in probability clouds rather than fixed orbits. Therefore, defining and measuring atomic radius becomes a nuanced endeavor.
Methods for Measuring Atomic Radius:
-
Covalent Radius: Determined by half the distance between the nuclei of two covalently bonded atoms of the same element. This method is suitable for nonmetals and metalloids, where covalent bonding dominates.
-
Metallic Radius: Measured as half the distance between the nuclei of two adjacent atoms in a metallic crystal. This method applies to metals where metallic bonding prevails.
-
Van der Waals Radius: Based on the distance between the nuclei of two non-bonded atoms in close proximity. This method accounts for the weak van der Waals forces that operate between non-polar molecules.
-
Theoretical Calculations: Quantum mechanical models and computational simulations can predict atomic radii with increasing accuracy. These methods offer a deeper understanding of the complex interplay of forces within the atom.
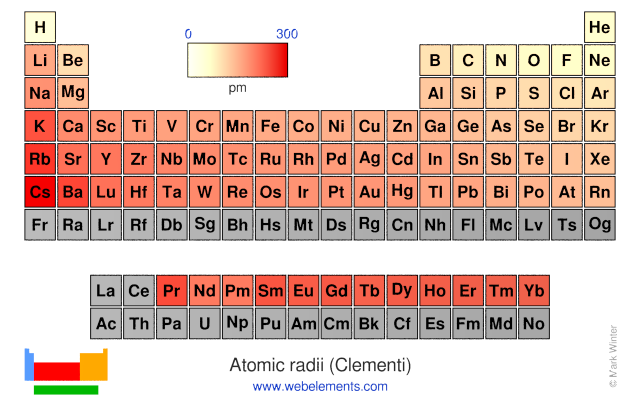
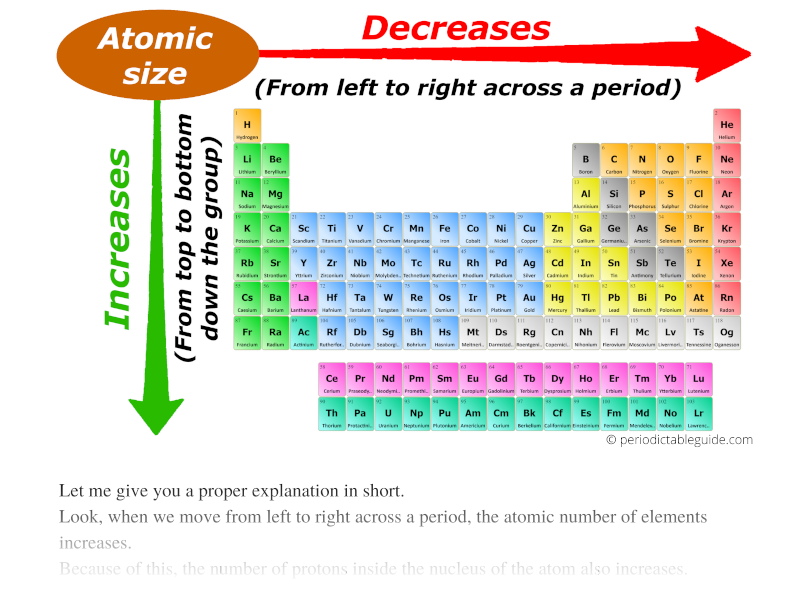
Trends in Atomic Radius: A Dance of Opposing Forces
As we navigate the periodic table, atomic radius exhibits predictable trends, governed by two opposing forces:
-
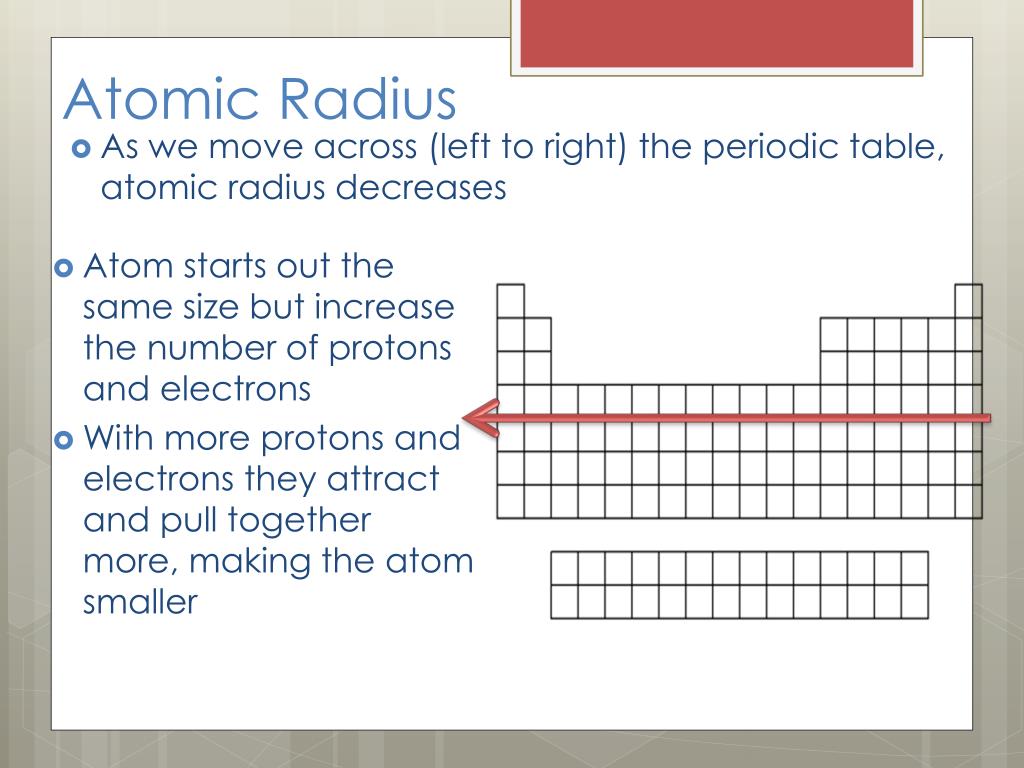
Effective Nuclear Charge (Zeff): The net positive charge experienced by an electron in an atom, considering the shielding effect of inner electrons. As we move across a period, Zeff increases due to the addition of protons without a corresponding increase in shielding electrons. This stronger attraction pulls the electrons closer to the nucleus, resulting in a decrease in atomic radius.
-
Principal Quantum Number (n): This quantum number determines the energy level and size of an electron shell. Moving down a group, the outermost electron occupies a higher energy level (higher n), leading to a larger atomic radius.
Understanding the Trends:
-
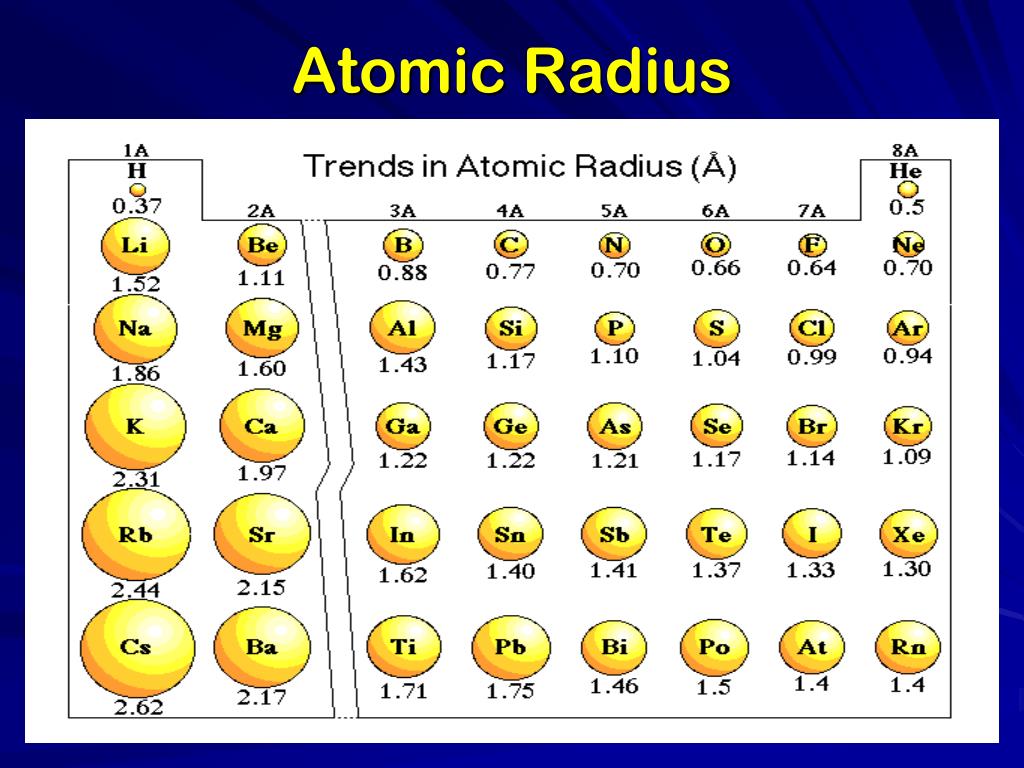
Across a Period: Atomic radius generally decreases from left to right. This is primarily due to the increasing Zeff, which pulls the electrons closer to the nucleus. For instance, lithium (Li) has a larger atomic radius than fluorine (F) because the electrons in lithium experience a weaker attraction to the nucleus.
-
Down a Group: Atomic radius increases from top to bottom. This is attributed to the increasing principal quantum number (n) of the outermost electron. As we move down a group, the electrons occupy higher energy levels, further away from the nucleus. For example, cesium (Cs) has a much larger atomic radius than lithium (Li) due to the higher energy levels occupied by its outermost electrons.
Exceptions to the Trends:
While the general trends hold true, certain exceptions exist, particularly in the transition metals. These exceptions arise from the complex interplay of factors like electron configuration and shielding effects. For instance, the atomic radius of copper (Cu) is slightly smaller than that of nickel (Ni), despite being located further down the group. This anomaly is attributed to the filled d-orbital in copper, which results in a stronger attraction between the nucleus and electrons.
Applications of Atomic Radius:
Understanding atomic radius is crucial in various fields:
-
Chemical Bonding: Atomic radii determine the bond lengths and bond angles in molecules, influencing their shape and reactivity.
-
Physical Properties: Atomic radius directly affects the density, melting point, and boiling point of elements. Larger atoms tend to have lower densities and melting points due to weaker interatomic forces.
-
Catalysis: The size of atoms plays a critical role in catalysis, determining the selectivity of catalysts and their ability to interact with specific molecules.
-
Materials Science: Atomic radius is essential for designing and developing new materials with desired properties. For instance, the size of atoms influences the properties of alloys, semiconductors, and nanomaterials.
Future Directions: Pushing the Boundaries of Atomic Radius
In 2025, the field of atomic radius research continues to advance. New techniques like X-ray diffraction with synchrotron radiation and advanced computational methods like density functional theory (DFT) provide more accurate and detailed information about atomic dimensions.
Key Areas of Research:
-
Relativistic Effects: For heavier elements, relativistic effects become increasingly significant, influencing electron distribution and atomic radius. Understanding these effects is crucial for accurate predictions of atomic properties.
-
Atomic Radius in Exotic Environments: Studying atomic radius in extreme conditions, such as high pressure or high temperature, provides insights into the behavior of matter under unusual circumstances.
-
Atomic Radius in Nanomaterials: The size of atoms plays a critical role in the properties of nanomaterials. Understanding atomic radius at the nanoscale is essential for designing and developing advanced materials with tailored properties.
Conclusion: A Fundamental Parameter with Profound Impact
The atomic radius, a seemingly simple concept, holds immense significance in our understanding of the chemical and physical world. By studying the trends in atomic radius, we gain valuable insights into the interplay of forces within atoms, paving the way for advancements in various fields, from materials science to medicine. As research continues to push the boundaries of our knowledge, the atomic radius will remain a fundamental parameter, driving innovation and shaping our future.
.png)