Reactivity Trends In The Periodic Table: A 2025 Perspective
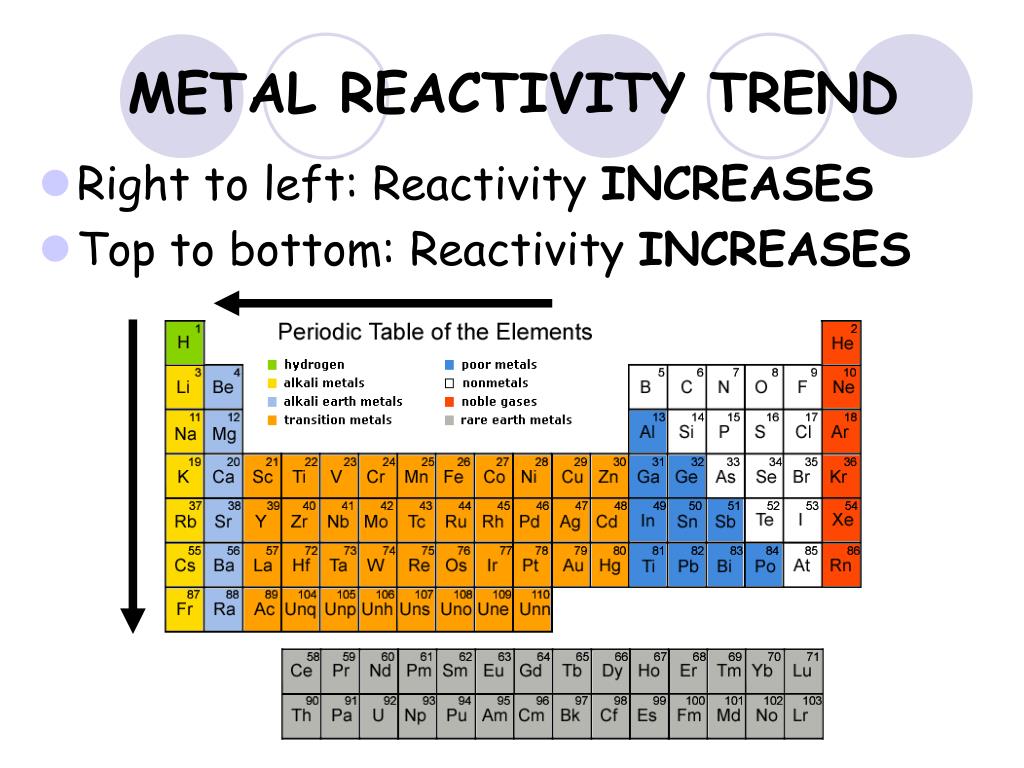
Reactivity Trends in the Periodic Table: A 2025 Perspective
The periodic table, a cornerstone of chemistry, continues to be a powerful tool for understanding and predicting the behavior of elements. As our understanding of the universe and its intricate chemical processes deepens, so too does our grasp of the reactivity trends that govern the periodic table. This article delves into the evolving landscape of reactivity trends in 2025, highlighting recent advancements and their implications for various fields.
1. Beyond the Basics: Unveiling Nuances in Reactivity
The traditional understanding of reactivity trends, based on electronegativity, ionization energy, and electron affinity, provides a foundational framework. However, contemporary research is revealing the intricate interplay of factors that influence reactivity, often leading to unexpected and fascinating behaviors.
a. The Role of Relativistic Effects:
Relativistic effects, particularly pronounced for heavier elements, are gaining increasing recognition for their impact on reactivity. These effects arise from the high speeds of electrons in heavier atoms, leading to changes in electron configurations and orbital energies. For instance, the relativistic contraction of the 6s orbital in gold (Au) contributes to its resistance to oxidation, explaining its inertness compared to other transition metals.
b. The Importance of Quantum Mechanics:
Advanced quantum mechanical calculations are becoming increasingly sophisticated, allowing for more precise predictions of reactivity. These calculations can account for complex interactions between electrons and nuclei, providing a deeper understanding of bond formation and reactivity patterns.
c. The Influence of Environment:
Reactivity is not solely an intrinsic property of an element but is also influenced by its environment. Factors like pressure, temperature, and the presence of other molecules can dramatically alter an element’s reactivity. For example, the inert gas xenon (Xe) can react with platinum (Pt) under high pressure, forming a stable compound.
2. Expanding Horizons: Uncovering New Reactivity Phenomena
The periodic table is not static; it is a dynamic system, constantly evolving with new discoveries and insights. Recent advancements have led to the identification of novel reactivity trends, challenging existing paradigms and opening up new avenues for exploration.
a. The Rise of Superheavy Elements:
The synthesis of superheavy elements, beyond uranium (U), has pushed the boundaries of the periodic table. These elements exhibit unique reactivity patterns, influenced by their highly unstable nuclei and relativistic effects. For example, oganesson (Og), the heaviest element currently known, is predicted to have a high electron affinity, potentially leading to its existence as a noble gas-like element.
b. The Intriguing World of Single-Atom Catalysis:
The development of single-atom catalysts, where individual metal atoms are dispersed on a support material, has revolutionized catalysis. These catalysts exhibit exceptional activity and selectivity due to their unique electronic properties and high surface area. This field is particularly relevant for renewable energy technologies, such as hydrogen production and carbon dioxide reduction.
c. The Promise of Main-Group Chemistry:
Main-group elements, often perceived as less reactive compared to transition metals, are now attracting renewed interest due to their unique properties and potential applications in various fields. For example, the development of highly efficient phosphine-based catalysts for organic synthesis is a testament to the growing importance of main-group chemistry.
3. Applications of Reactivity Trends: Shaping the Future
Understanding reactivity trends is not merely an academic pursuit; it has profound implications for diverse fields, driving innovation and shaping the future.
a. Materials Science and Engineering:
Reactivity trends guide the development of novel materials with tailored properties. For instance, the understanding of the reactivity of different metals with oxygen has led to the creation of corrosion-resistant alloys for aerospace and automotive applications.
b. Medicine and Drug Discovery:
The reactivity of specific functional groups within molecules is crucial for drug design and development. Understanding these trends allows for the creation of drugs that selectively target specific biological pathways, enhancing therapeutic efficacy and minimizing side effects.
c. Environmental Science and Sustainability:
Reactivity trends play a critical role in understanding and mitigating environmental issues. For example, the reactivity of heavy metals like mercury (Hg) and lead (Pb) with various environmental components helps us understand their fate and transport in the environment, allowing for more effective remediation strategies.
4. Challenges and Future Directions
Despite the remarkable progress in understanding reactivity trends, several challenges remain:
a. Predicting Reactivity in Complex Systems:
Accurately predicting reactivity in complex systems, such as biological environments or industrial processes, is a significant challenge. The interplay of multiple factors, including temperature, pressure, and the presence of other molecules, makes it difficult to develop comprehensive models.
b. Bridging the Gap between Theory and Experiment:
While quantum mechanical calculations provide valuable insights, bridging the gap between theoretical predictions and experimental observations remains a key challenge. This gap arises from the limitations of current computational methods and the difficulty in replicating real-world conditions in laboratory settings.
c. Harnessing the Power of Machine Learning:
Machine learning is emerging as a powerful tool for analyzing vast datasets and identifying complex patterns. The application of machine learning to reactivity prediction holds immense potential for accelerating discovery and innovation.
5. Looking Ahead: A Vision for the Future
The future of reactivity trends research is bright, driven by the continued development of advanced experimental techniques, computational methods, and data-driven approaches.
a. Developing Novel Experimental Techniques:
New experimental techniques, such as femtosecond spectroscopy and atomic force microscopy, will allow for real-time observation of chemical reactions at the atomic level, providing unprecedented insights into reactivity mechanisms.
b. Leveraging High-Performance Computing:
The increasing availability of high-performance computing resources will enable more sophisticated quantum mechanical calculations, allowing for more accurate predictions of reactivity in complex systems.
c. Harnessing the Power of Artificial Intelligence:
Artificial intelligence (AI) will play an increasingly significant role in reactivity research, enabling the development of intelligent systems that can predict reactivity, design new materials, and optimize chemical processes.
Conclusion
The periodic table, a timeless symbol of chemical organization, continues to evolve as our understanding of reactivity deepens. This evolution is driven by the interplay of experimental observations, theoretical calculations, and emerging technologies. As we delve deeper into the intricacies of reactivity, we unlock new possibilities for innovation and progress in various fields, from materials science and medicine to environmental science and beyond. The future of reactivity trends research promises to be exciting, filled with discoveries that will shape our world in profound ways.
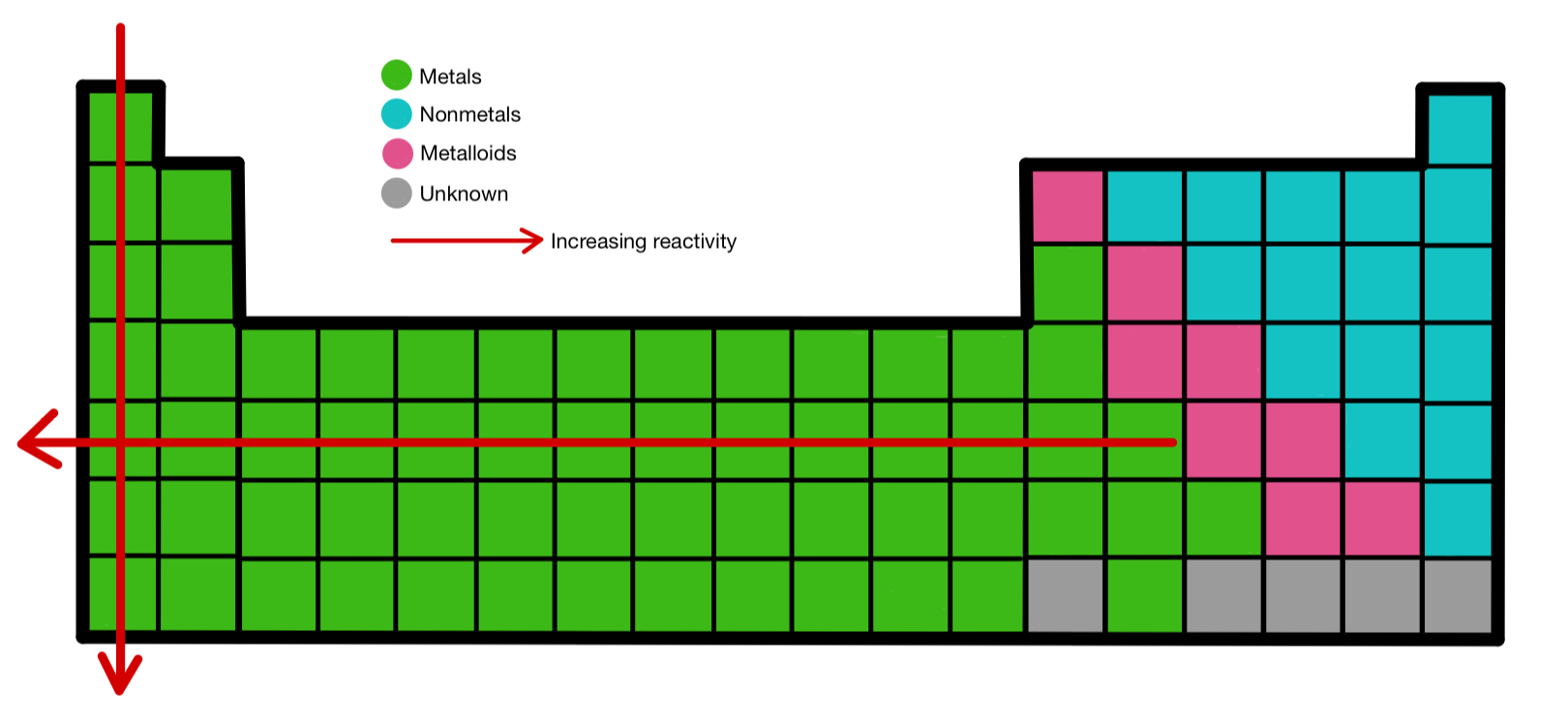
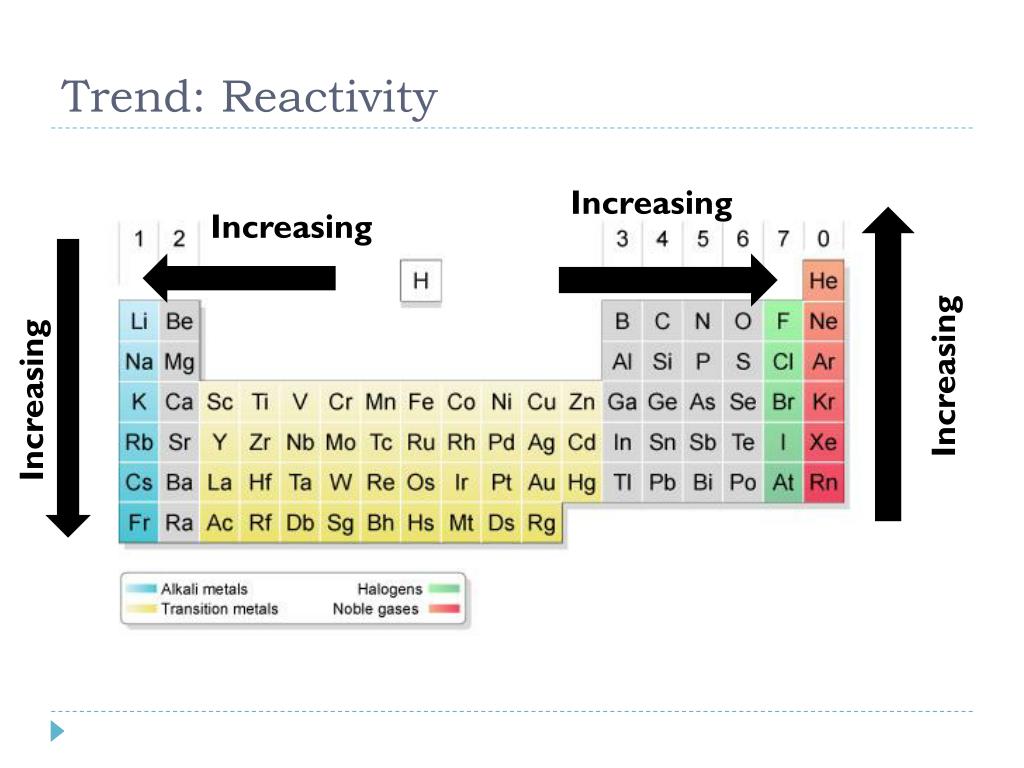

:max_bytes(150000):strip_icc()/chart-of-periodic-table-trends-608792-v1-6ee35b80170349e8ab67865a2fdfaceb.png)
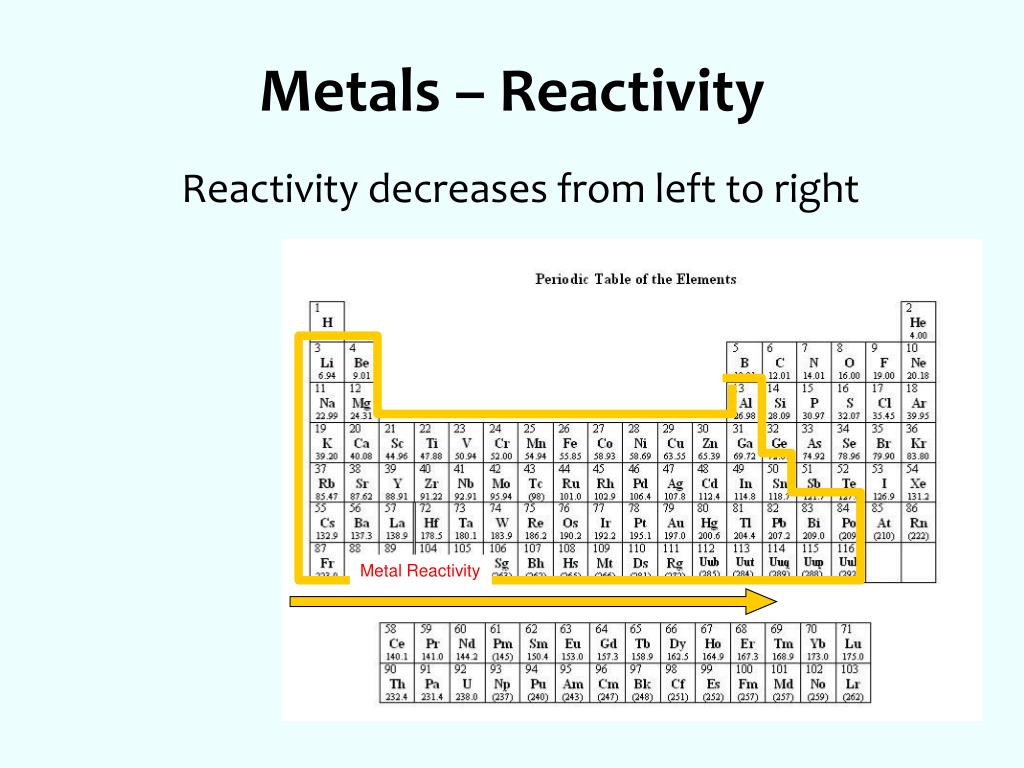

![Chemistry Chemical Reactivity Lab: Periodic Trends - [PPT Powerpoint]](https://cdn.vdocuments.mx/img/1200x630/reader021/image/20170828/56649e245503460f94b12897.png?t=1603128099)
/periodictrendstable-5c4a46614cedfd000187c5db.jpg)