Reactivity Trends In The Periodic Table: A 2025 Perspective
Reactivity Trends in the Periodic Table: A 2025 Perspective
The periodic table, a cornerstone of chemistry, provides a framework for understanding the behavior of elements and their interactions. One of the most fundamental concepts in chemistry is reactivity, which describes how readily an element undergoes chemical reactions. While the basic trends in reactivity are well-established, ongoing research continues to refine our understanding and uncover new insights. This article delves into the current state of knowledge regarding reactivity trends in the periodic table, focusing on advancements made since 2020, and explores the potential for future discoveries.
Understanding Reactivity: A Recap
Reactivity is influenced by a complex interplay of factors, including:
- Electronegativity: The ability of an atom to attract electrons towards itself. Elements with high electronegativity tend to gain electrons, forming anions, while those with low electronegativity tend to lose electrons, forming cations.
- Ionization Energy: The energy required to remove an electron from a gaseous atom. Elements with low ionization energies readily lose electrons, becoming cations, while those with high ionization energies resist electron loss.
- Electron Affinity: The change in energy when an electron is added to a neutral atom in the gaseous state. Elements with high electron affinity readily gain electrons, forming anions.
- Atomic Radius: The size of an atom. Smaller atoms have a greater attraction for electrons due to the closer proximity of the nucleus, influencing reactivity.
- Metallic Character: The tendency of an element to lose electrons and form positive ions. Metals generally have high reactivity.
Reactivity Trends Across the Periodic Table
1. Metals:
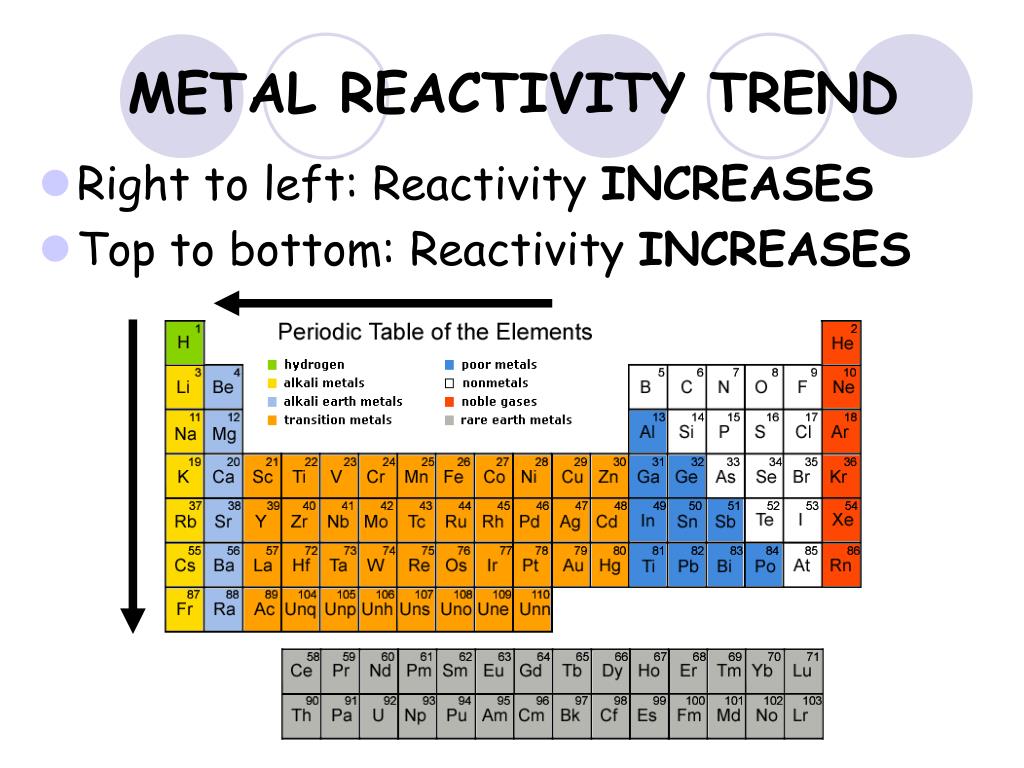
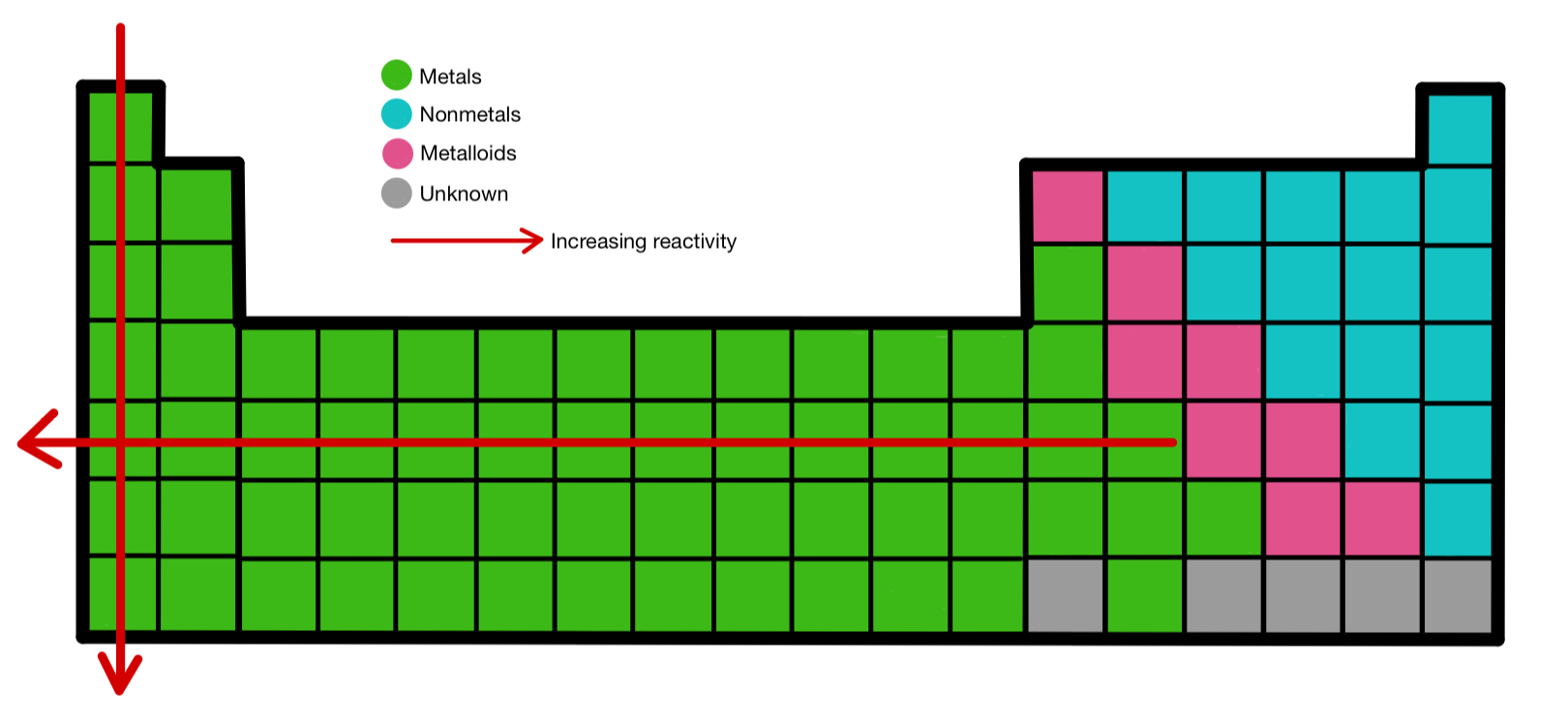
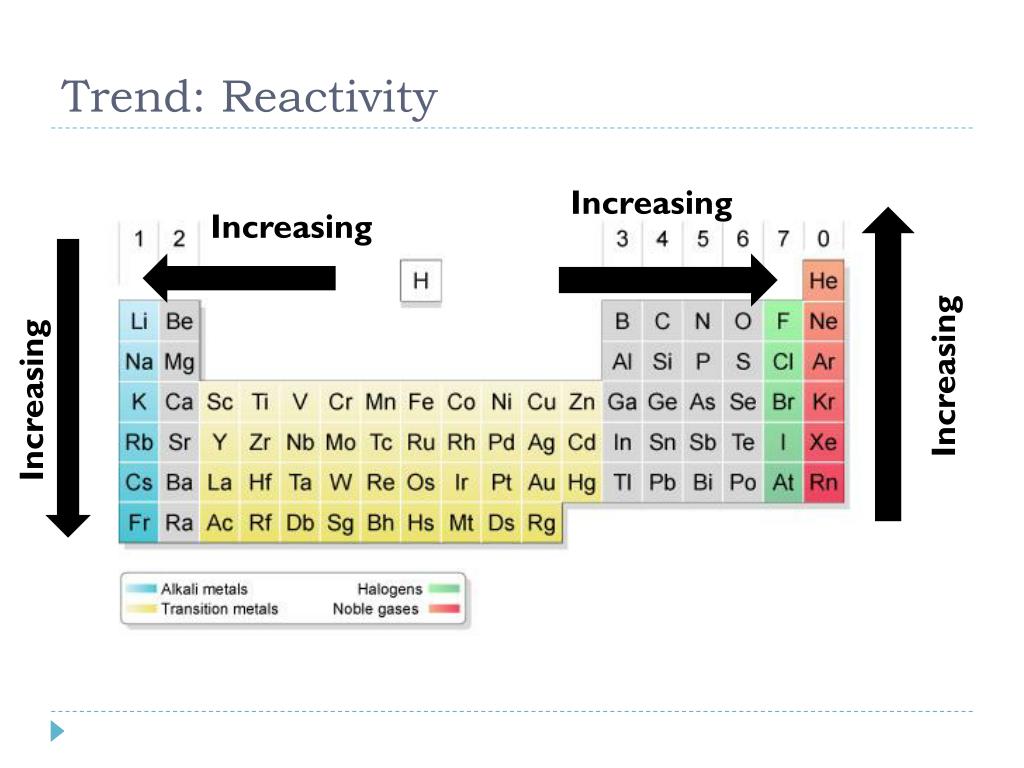
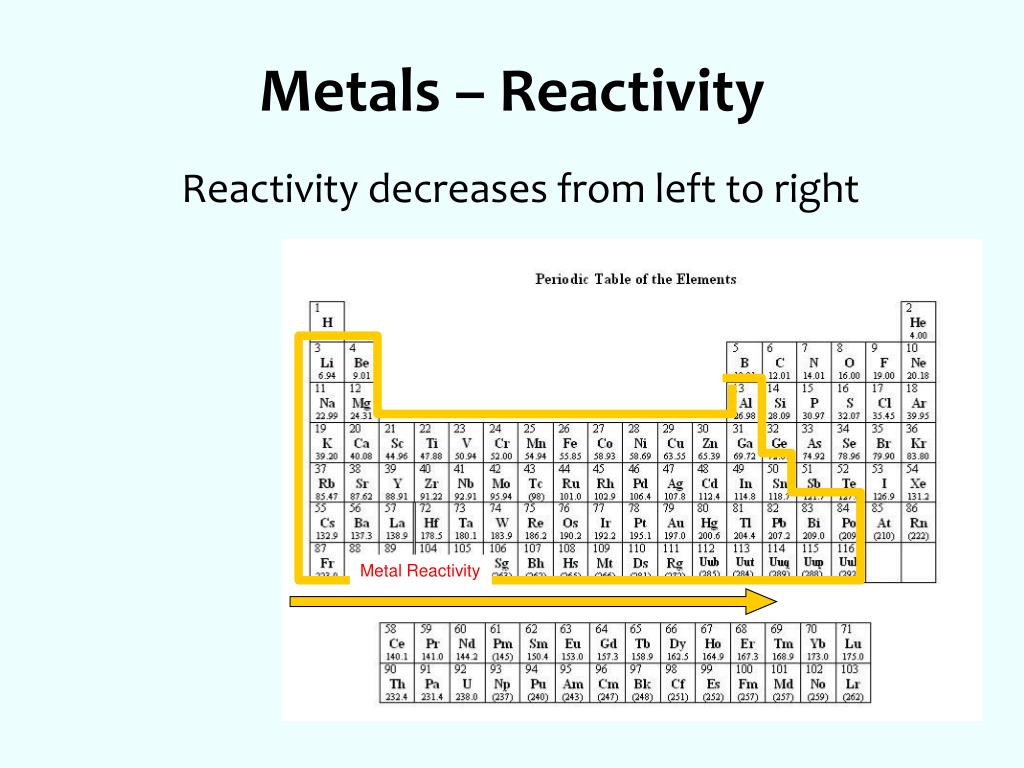
- Group 1 (Alkali Metals): These elements are highly reactive, readily losing their single valence electron to form +1 cations. Reactivity increases down the group due to decreasing ionization energy and increasing atomic radius. This trend is exemplified by lithium’s relatively low reactivity compared to the highly reactive cesium.
- Group 2 (Alkaline Earth Metals): Similar to alkali metals, alkaline earth metals exhibit high reactivity, losing two valence electrons to form +2 cations. Reactivity also increases down the group, with beryllium being less reactive than barium.
- Transition Metals: Transition metals exhibit a wide range of reactivity, influenced by factors like electron configuration and oxidation states. Some, like gold and platinum, are relatively unreactive, while others like iron and copper show significant reactivity. Their reactivity often depends on specific conditions and the presence of other elements.
2. Nonmetals:
- Group 17 (Halogens): Halogens are highly reactive nonmetals, readily gaining one electron to form -1 anions. Reactivity decreases down the group due to increasing atomic radius and decreasing electronegativity. Fluorine, the most electronegative element, is the most reactive halogen.
- Group 16 (Chalcogens): Chalcogens exhibit varying reactivity, with oxygen being the most reactive. Reactivity decreases down the group due to decreasing electronegativity.
- Group 15 (Pnictogens): Pnictogens show diverse reactivity, influenced by their electron configurations and oxidation states. Nitrogen, with its strong triple bond, is relatively unreactive, while phosphorus and arsenic exhibit greater reactivity.
- Group 14 (Carbon Group): Carbon, the cornerstone of organic chemistry, exhibits unique reactivity due to its ability to form multiple bonds. Silicon, the second member of the group, is used extensively in semiconductors due to its controlled reactivity.
- Group 13 (Boron Group): Boron, the first member of the group, is a metalloid with unique reactivity, forming covalent bonds with other elements.
Recent Advancements in Understanding Reactivity
1. Role of Quantum Mechanics:
Recent years have seen a significant advancement in understanding reactivity through the lens of quantum mechanics. Computational chemistry, aided by powerful algorithms and increased computing power, allows researchers to predict and analyze chemical reactions at the atomic level. This approach provides insights into the electronic structure and bonding characteristics of molecules, leading to a more precise understanding of reactivity patterns.
2. Exploring the Reactivity of Superheavy Elements:
The synthesis and characterization of superheavy elements, elements beyond uranium, have opened new frontiers in understanding reactivity. These elements are highly unstable and short-lived, making their study challenging. However, theoretical calculations and limited experimental data have revealed interesting trends. For example, studies suggest that some superheavy elements may exhibit unexpected reactivity due to relativistic effects, where the high speed of electrons near the nucleus alters their behavior.
3. Unveiling the Reactivity of Novel Materials:
The development of new materials, such as nanomaterials, metal-organic frameworks (MOFs), and 2D materials like graphene, has led to the exploration of their unique reactivity properties. Nanomaterials, with their high surface area and quantum effects, often exhibit enhanced reactivity compared to their bulk counterparts. MOFs, with their porous structures and tunable properties, offer potential for selective catalysis and gas adsorption. Graphene, a single layer of carbon atoms, exhibits exceptional electrical conductivity and mechanical strength, making it attractive for applications in electronics and energy storage.
4. Uncovering the Reactivity of Exotic Species:
Research into the reactivity of exotic species, such as free radicals, carbenes, and reactive oxygen species (ROS), has deepened our understanding of chemical processes in biological systems and environmental chemistry. Free radicals, with their unpaired electrons, are highly reactive and play a critical role in aging, disease, and atmospheric chemistry. Carbenes, containing a divalent carbon atom, are highly reactive intermediates involved in organic synthesis and photochemistry. ROS, including superoxide and hydroxyl radicals, are implicated in cellular damage and inflammation.
Future Directions in Reactivity Research
1. Predictive Modeling of Reactivity:
The development of more sophisticated computational models, capable of accurately predicting reactivity across the periodic table, remains a key focus. These models will be instrumental in designing new materials with desired properties and optimizing existing chemical processes.
2. Exploring the Reactivity of Superheavy Elements:
Further research on the reactivity of superheavy elements is crucial to understanding the behavior of matter at the limits of the periodic table. This research will require the development of new techniques for synthesizing and characterizing these elements, as well as advanced theoretical models to account for relativistic effects.
3. Understanding the Reactivity of Novel Materials:
Continued exploration of the reactivity of novel materials, such as nanomaterials, MOFs, and 2D materials, will lead to the development of new technologies for catalysis, energy storage, and environmental remediation.
4. Elucidating the Reactivity of Exotic Species:
Further research into the reactivity of exotic species will enhance our understanding of fundamental chemical processes and their implications in biological systems, environmental chemistry, and materials science.
Conclusion
The study of reactivity trends in the periodic table is an ongoing journey of discovery and refinement. Advancements in computational chemistry, the synthesis of superheavy elements, the exploration of novel materials, and the investigation of exotic species are continuously expanding our knowledge of chemical behavior. As we delve deeper into the intricacies of atomic interactions, we unlock new possibilities for developing innovative technologies and solving critical challenges facing our world. The periodic table, with its rich tapestry of elements, continues to be a source of inspiration and wonder, guiding us towards a more profound understanding of the fundamental principles that govern the universe.

:max_bytes(150000):strip_icc()/chart-of-periodic-table-trends-608792-v1-6ee35b80170349e8ab67865a2fdfaceb.png)

![Chemistry Chemical Reactivity Lab: Periodic Trends - [PPT Powerpoint]](https://cdn.vdocuments.mx/img/1200x630/reader021/image/20170828/56649e245503460f94b12897.png?t=1603128099)
/periodictrendstable-5c4a46614cedfd000187c5db.jpg)