Ionization Energy Trends In The Periodic Table: A 2025 Perspective
Ionization Energy Trends in the Periodic Table: A 2025 Perspective
The periodic table, a cornerstone of chemistry, reveals intricate patterns in the properties of elements. One such property, ionization energy, plays a crucial role in understanding chemical reactivity and bonding. Ionization energy, defined as the minimum energy required to remove an electron from a gaseous atom or ion in its ground electronic state, offers a window into the forces holding electrons within an atom’s grasp.
As we delve deeper into the realm of chemical science, our understanding of ionization energy continues to evolve. This article explores the trends of ionization energy in the periodic table, highlighting key insights from the year 2025.
Understanding the Basics: The Dance of Electrons and Energy
Ionization energy is a fundamental concept in atomic physics and chemistry. It quantifies the strength of the electrostatic attraction between the nucleus and its electrons. The higher the ionization energy, the more tightly bound the electron is to the atom, making it more difficult to remove. This energy is typically measured in electron volts (eV) or kilojoules per mole (kJ/mol).
Periodic Trends: A Symphony of Atomic Structure
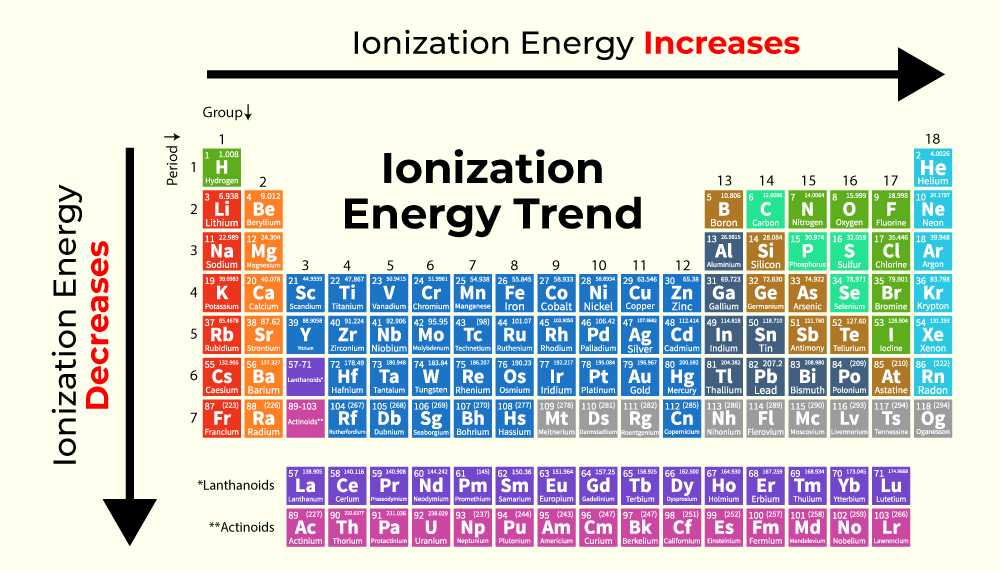
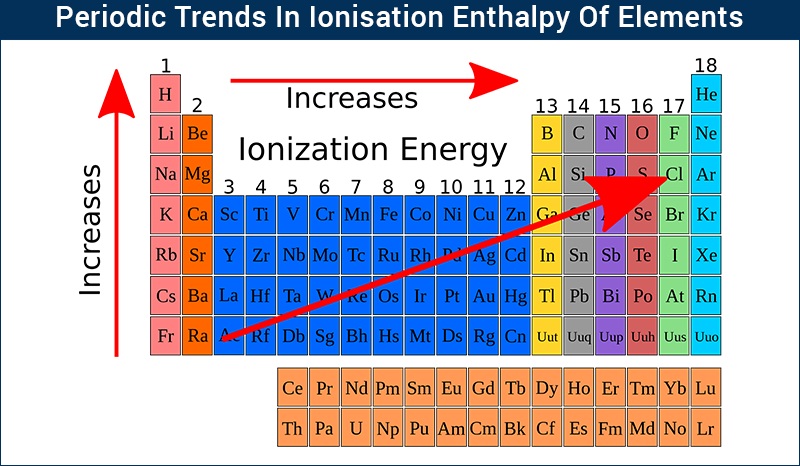
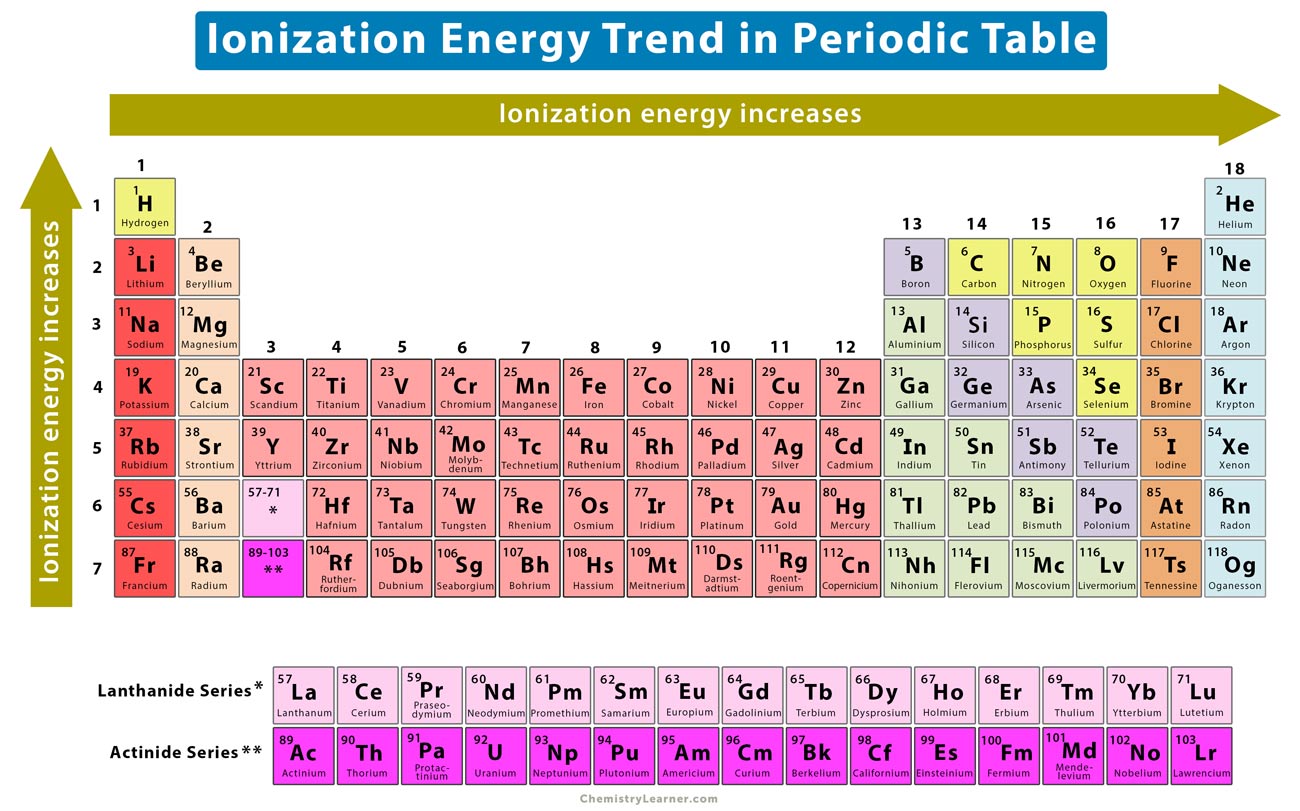
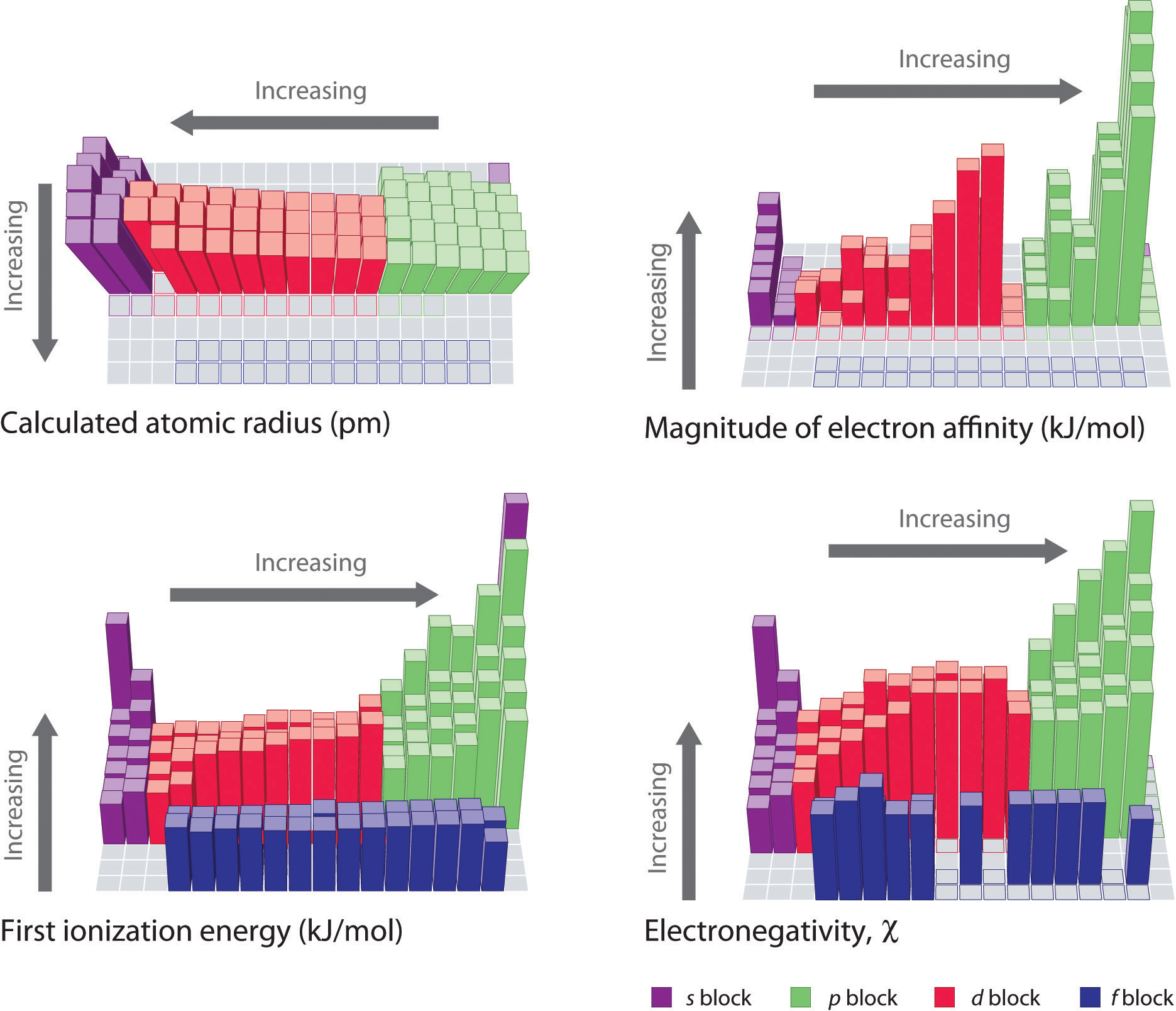
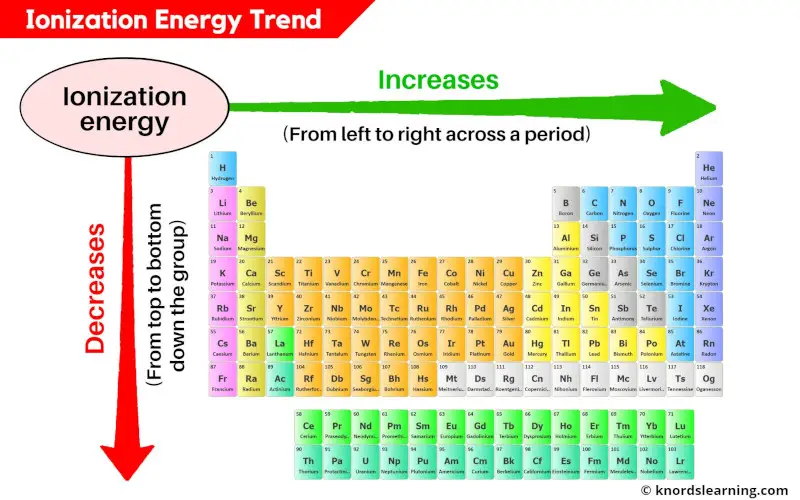
The periodic table, with its organized arrangement of elements, unveils fascinating trends in ionization energy. These trends are a direct consequence of the interplay between atomic size, nuclear charge, and electron configuration.
Across a Period:
-
Increasing Ionization Energy: As we move from left to right across a period, ionization energy generally increases. This is because the number of protons in the nucleus increases, leading to a stronger attraction between the nucleus and the electrons. The electrons in the same energy level experience an increased effective nuclear charge, pulling them closer to the nucleus and making them harder to remove.
-
Shielding Effect: The core electrons, those closer to the nucleus, shield the outer valence electrons from the full nuclear charge. This shielding effect is relatively constant across a period, as the number of core electrons remains the same.
-
Exceptions: Some exceptions to this trend arise due to the presence of half-filled and completely filled subshells. For instance, the ionization energy of nitrogen is slightly higher than that of oxygen. This is because nitrogen has a half-filled p-subshell, which provides extra stability.
Down a Group:
-
Decreasing Ionization Energy: As we move down a group, ionization energy generally decreases. This is primarily due to the increasing atomic size. The valence electrons are further away from the nucleus, experiencing a weaker attraction.
-
Increased Shielding: The increased number of electron shells in heavier elements leads to greater shielding of the valence electrons by the core electrons. This further weakens the attraction between the nucleus and the valence electrons.
Factors Influencing Ionization Energy
Several factors, beyond the basic periodic trends, play a role in determining ionization energy. These include:
-
Electron Configuration: The stability of electron configurations, such as half-filled or completely filled subshells, can influence ionization energy. Atoms with stable configurations tend to have higher ionization energies.
-
Electron-Electron Repulsion: Electrons in the same energy level repel each other. This repulsion weakens the attraction between the nucleus and the valence electrons, lowering ionization energy.
-
Penetration Effect: The penetration effect describes the ability of an electron to approach the nucleus. Electrons in s-orbitals, for example, have a higher penetration effect than electrons in p-orbitals, leading to stronger attraction to the nucleus and higher ionization energy.
Applications of Ionization Energy
Ionization energy is a fundamental concept with numerous applications across diverse scientific fields. Here are a few key examples:
-
Predicting Chemical Reactivity: Elements with low ionization energies tend to be more reactive, readily losing electrons to form cations. Conversely, elements with high ionization energies are less reactive, holding onto their electrons tightly.
-
Understanding Chemical Bonding: The difference in ionization energies between two atoms can provide insights into the type of bond they will form. For instance, a large difference in ionization energies suggests an ionic bond, while a smaller difference suggests a covalent bond.
-
Spectroscopy: Ionization energy is a crucial parameter in various spectroscopic techniques, such as photoelectron spectroscopy, which provides information about the energy levels of electrons in atoms and molecules.
-
Materials Science: Ionization energy plays a role in understanding the electronic properties of materials. For instance, materials with low ionization energies are often used in solar cells and organic electronics, where electron transfer is critical.
2025: A Year of Innovation and Discovery
As we progress towards 2025, the field of ionization energy continues to evolve. Advancements in computational chemistry and experimental techniques are enabling us to explore ionization energy with unprecedented precision and detail.
Here are some key trends shaping our understanding of ionization energy in 2025:
-
Computational Modeling: Sophisticated computational models, powered by quantum mechanics, are becoming increasingly accurate in predicting ionization energies. These models allow us to study complex systems and explore the effects of various factors on ionization energy.
-
High-Resolution Spectroscopy: Advanced spectroscopic techniques, such as high-resolution photoelectron spectroscopy, are providing increasingly detailed information about ionization energies. These techniques enable us to probe the fine structure of electron energy levels and gain a deeper understanding of electron-electron interactions.
-
Ionization Energy in Exotic Systems: Researchers are exploring ionization energies in exotic systems, such as nanoparticles, clusters, and even individual atoms trapped in optical lattices. These studies provide insights into the fundamental nature of ionization and its role in the behavior of matter at the nanoscale.
-
Applications in Emerging Technologies: Ionization energy is playing a crucial role in the development of emerging technologies, such as solar energy, quantum computing, and advanced materials. Understanding ionization energy is essential for designing materials with specific electronic properties and optimizing the efficiency of these technologies.
Looking Ahead: The Future of Ionization Energy
The study of ionization energy is a dynamic field with exciting prospects for the future. As we continue to refine our theoretical models and develop new experimental techniques, we can expect to gain a deeper understanding of this fundamental property and its role in shaping the world around us.
Here are some key areas where research on ionization energy is expected to make significant progress in the years to come:
-
Relativistic Effects: For heavy elements, relativistic effects become increasingly significant, influencing electron energies and ionization potentials. Further research into these effects is crucial for understanding the behavior of heavy elements.
-
Non-Equilibrium Ionization: Traditional ionization energy measurements focus on equilibrium conditions. However, in many real-world applications, ionization occurs under non-equilibrium conditions, such as in plasmas or during chemical reactions. Understanding ionization energy in these scenarios is crucial for various applications.
-
Ionization Energy in Complex Systems: The study of ionization energy is expanding beyond isolated atoms to encompass complex systems like molecules, clusters, and extended solids. Understanding ionization energy in these systems is essential for developing new materials and technologies.
-
Ionization Energy in Astrochemistry: Ionization energy plays a crucial role in astrochemical processes, such as the formation of interstellar molecules. Studying ionization energy in the context of astrochemistry can provide insights into the origins of life and the evolution of the universe.
Conclusion: A Journey of Exploration
The study of ionization energy is a continuous journey of exploration. From its fundamental role in atomic structure to its applications in diverse fields, ionization energy remains a cornerstone of our understanding of chemistry and the world around us. As we continue to delve deeper into the intricacies of this property, we can expect to unlock new insights and discover exciting applications that will shape the future of science and technology.
.PNG)