Ionization Energy Trends In 2025: A Look At The Future Of Atomic Physics
Ionization Energy Trends in 2025: A Look at the Future of Atomic Physics
The year 2025 marks a significant point in the evolution of atomic physics, particularly in the field of ionization energy. As our understanding of atomic structure deepens and experimental techniques advance, we are witnessing a dynamic shift in how we perceive and utilize this fundamental property. This article delves into the predicted trends in ionization energy research, exploring the potential breakthroughs, challenges, and applications that lie ahead.
The Foundation: Ionization Energy and its Significance
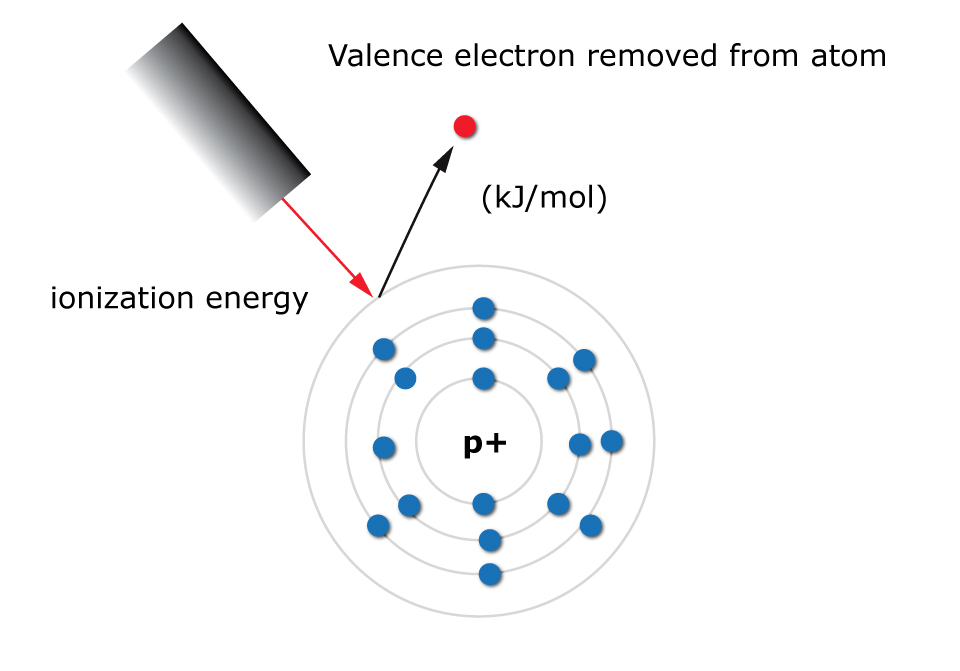
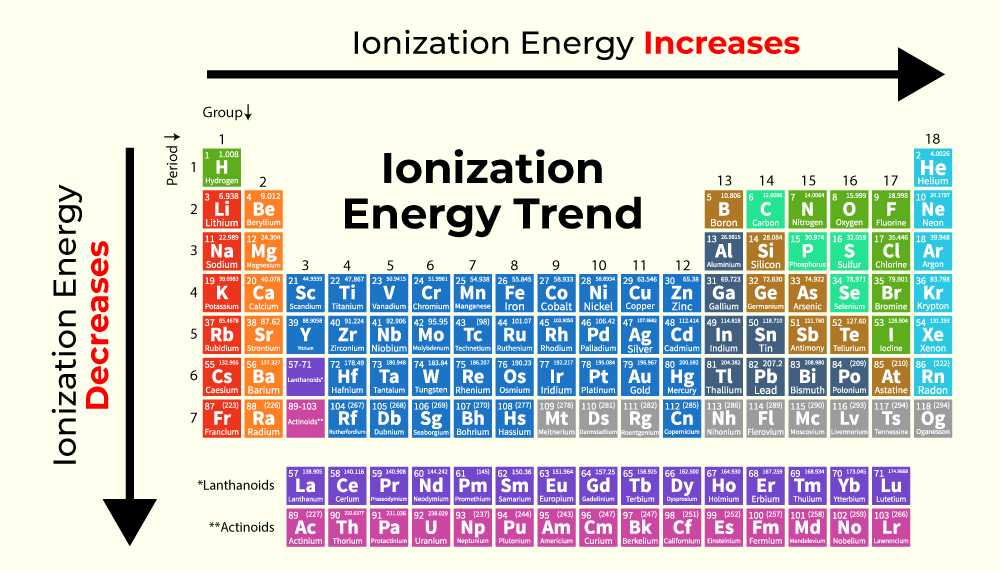
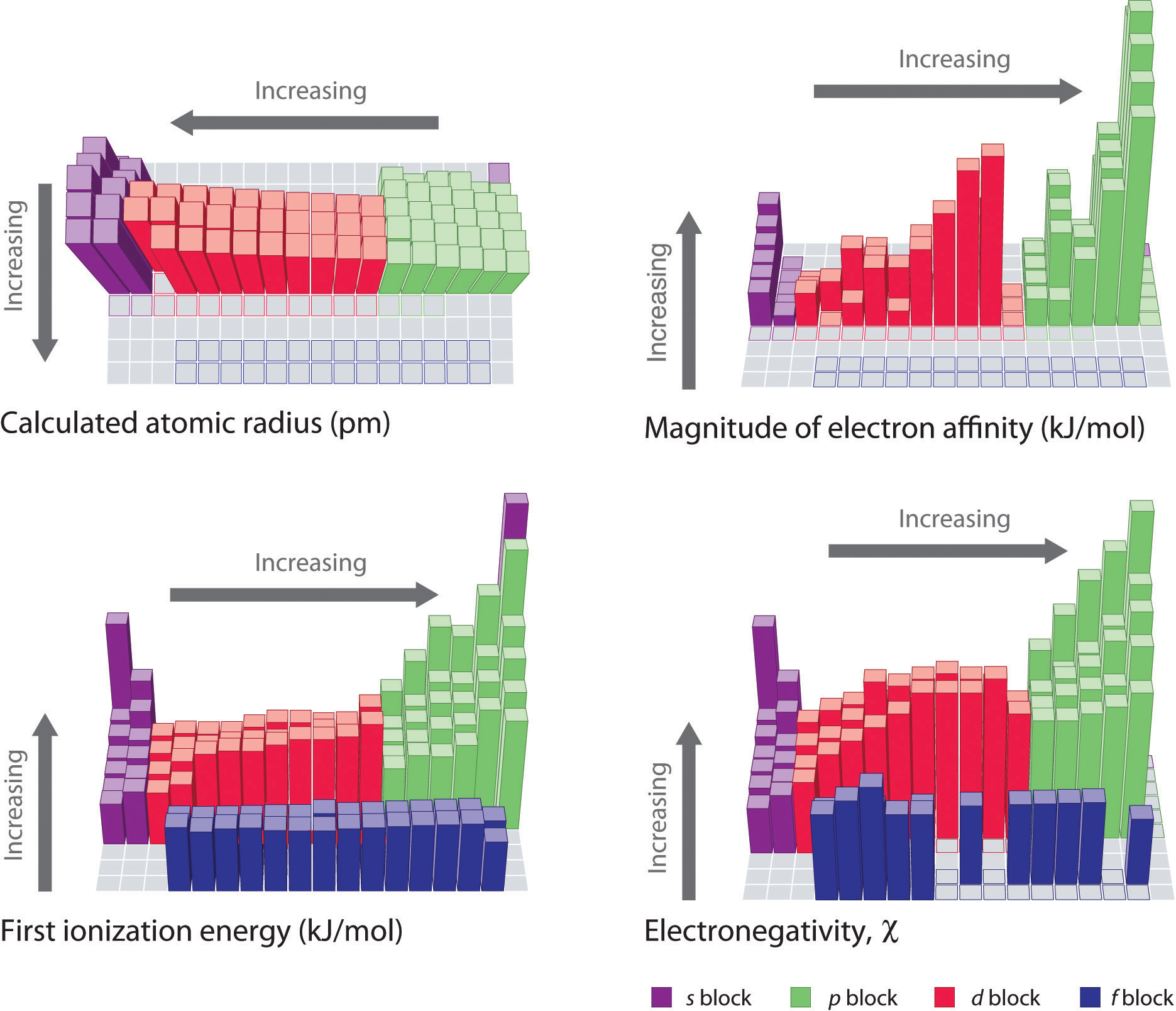
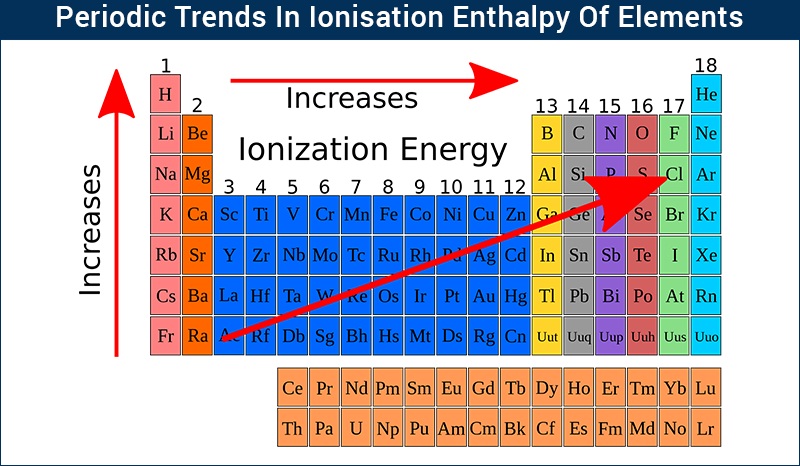
Ionization energy, the minimum energy required to remove an electron from an atom in its gaseous state, is a cornerstone of atomic physics. It dictates an atom’s reactivity, its role in chemical bonding, and its behavior in various physical phenomena. Understanding ionization energy allows us to predict and control chemical reactions, design novel materials, and develop advanced technologies.
Trends Shaping the Future of Ionization Energy Research
The landscape of ionization energy research is undergoing a transformation, driven by several key trends:
1. Precision and Accuracy: Pushing the Boundaries of Measurement
The quest for ever-greater precision in ionization energy measurements is a constant pursuit. Advanced spectroscopic techniques, including photoelectron spectroscopy and laser-based methods, are being refined to achieve unprecedented accuracy. This drive for precision is fueled by the desire to:
- Validate theoretical models: Accurate ionization energy data serves as a benchmark for testing and refining theoretical models of atomic structure and electron-electron interactions.
- Unlock new insights: Subtle variations in ionization energy can reveal crucial information about the electronic structure, electron correlations, and relativistic effects within atoms.
- Enhance technological applications: Precise ionization energy data is crucial for designing highly sensitive analytical techniques, like mass spectrometry, and for developing advanced materials with tailored properties.
2. Beyond the Ground State: Probing Excited States and Ions
Traditional ionization energy studies focus on the removal of an electron from the ground state of an atom. However, research is expanding to encompass excited states and ions. This shift is motivated by:
- Unveiling the complexity of excited states: Excited states play a crucial role in various physical processes, including light emission, energy transfer, and chemical reactions. Understanding their ionization energies provides valuable insights into their behavior and reactivity.
- Exploring the dynamics of ions: Ions are ubiquitous in various environments, from interstellar space to the human body. Studying their ionization energies helps us understand their interactions, their role in chemical reactions, and their behavior in plasmas.
- Developing novel technologies: Ionization energies of excited states and ions hold potential for developing new technologies, including lasers, plasma diagnostics, and advanced materials.
3. The Power of Computational Methods: Bridging Theory and Experiment
Computational chemistry and physics have emerged as powerful tools for predicting and interpreting ionization energies. Advanced quantum chemical methods, like density functional theory (DFT) and coupled cluster theory, are being employed to:
- Calculate ionization energies: Computational methods can predict ionization energies with increasing accuracy, offering valuable insights into the electronic structure and bonding properties of atoms and molecules.
- Interpret experimental results: Computational simulations can aid in interpreting experimental data and provide deeper understanding of the underlying physical processes.
- Explore complex systems: Computational methods can be applied to study ionization energies of large molecules, clusters, and even solids, which are challenging to study experimentally.
4. From Atoms to Molecules: Expanding the Scope of Ionization Energy
While traditionally focused on atoms, ionization energy research is now expanding to encompass molecules. This shift is driven by the need to:
- Understand molecular bonding: Ionization energies of molecules provide crucial information about the strength and nature of chemical bonds, offering insights into molecular stability and reactivity.
- Develop new materials: Understanding ionization energies of molecules is crucial for designing new materials with tailored properties, including conductivity, optical properties, and catalytic activity.
- Explore biological systems: Ionization energies of biomolecules play a vital role in biological processes, such as photosynthesis, DNA replication, and enzyme catalysis.
5. Ionization Energy in the Context of Emerging Technologies
Ionization energy is emerging as a key parameter in the development of several cutting-edge technologies:
- Plasma physics and fusion energy: Ionization energy is crucial for understanding plasma behavior and optimizing conditions for fusion reactions.
- Laser-based technologies: Ionization energy plays a crucial role in the development of advanced lasers, including attosecond lasers, used in high-precision spectroscopy and ultrafast dynamics studies.
- Materials science: Ionization energy is a crucial parameter for designing new materials with specific electronic properties, including conductivity, optical properties, and catalytic activity.
- Nanotechnology: Understanding ionization energies of nanomaterials is essential for controlling their properties and developing novel applications in electronics, sensors, and energy storage.
Challenges and Opportunities
While the future of ionization energy research holds immense promise, several challenges need to be addressed:
- Computational complexity: Accurately calculating ionization energies for complex systems, such as large molecules or condensed phases, remains computationally demanding.
- Experimental limitations: Measuring ionization energies of excited states and ions with high precision can be experimentally challenging.
- Interdisciplinary collaborations: Solving complex problems in ionization energy research requires strong collaborations between experimentalists, theorists, and researchers from diverse fields.
These challenges also present opportunities for innovation and progress:
- Developing novel computational methods: The development of more efficient and accurate computational methods is crucial for tackling complex systems and achieving higher predictive power.
- Improving experimental techniques: Continued refinement of experimental techniques, including laser-based methods and advanced spectroscopic techniques, is essential for achieving greater precision and exploring new frontiers.
- Fostering interdisciplinary collaborations: Encouraging collaborations between physicists, chemists, and material scientists will lead to a more comprehensive understanding of ionization energy and its applications.
Looking Ahead: The Impact of Ionization Energy Research
The future of ionization energy research holds immense potential for advancing our understanding of the fundamental building blocks of matter and for developing transformative technologies. By pushing the boundaries of precision, exploring new frontiers in excited states and ions, harnessing the power of computational methods, and expanding the scope of ionization energy research to molecules and beyond, we can unlock a wealth of new knowledge and applications.
Examples of Potential Applications:
- Developing novel catalysts: Understanding ionization energies of molecules can lead to the design of highly efficient catalysts for various chemical reactions, including those involved in energy production and environmental remediation.
- Creating advanced materials: Ionization energies play a crucial role in the development of materials with tailored electronic properties, such as conductivity, optical properties, and magnetic behavior. These materials can find applications in electronics, optics, and energy storage.
- Improving medical diagnostics: Ionization energy measurements can be utilized in advanced medical imaging techniques, allowing for more accurate and sensitive detection of diseases.
- Exploring the universe: Understanding ionization energies of atoms and molecules is essential for studying the composition and evolution of stars, planets, and interstellar space.
Conclusion
The year 2025 marks a turning point in ionization energy research, a field poised for significant advancements. By embracing the trends of precision, expansion, and interdisciplinarity, we can unlock the full potential of this fundamental property, paving the way for groundbreaking discoveries and transformative technologies. The future of ionization energy research promises to be both exciting and impactful, shaping our understanding of the universe and driving innovation across diverse fields.