Ionization Energy: A Periodic Journey Through The Elements In 2025
Ionization Energy: A Periodic Journey Through the Elements in 2025
The periodic table, a cornerstone of chemistry, provides a framework for understanding the behavior of elements. Among the many properties that vary predictably across the table, ionization energy stands out as a crucial indicator of an atom’s tendency to lose electrons and form cations. As we delve into the year 2025, our understanding of ionization energy continues to evolve, driven by advancements in theoretical modeling, experimental techniques, and the pursuit of novel applications.
A Fundamental Concept: What is Ionization Energy?
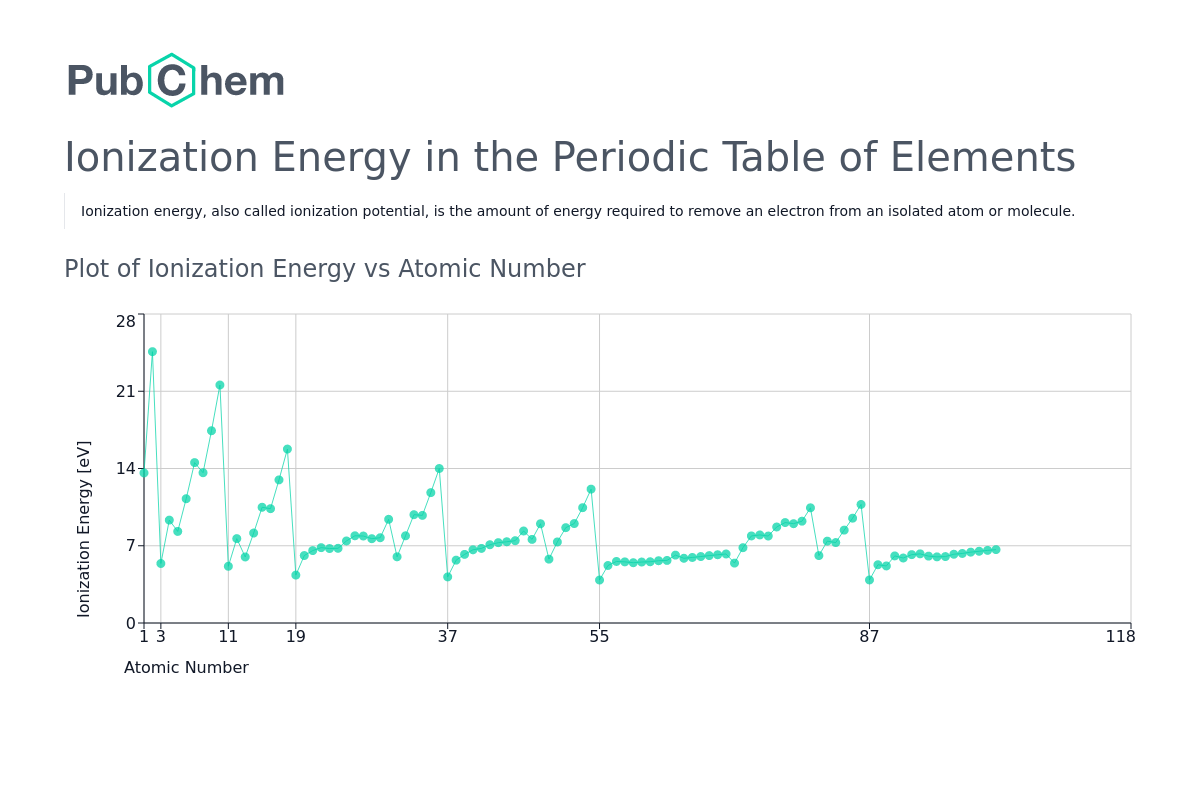
Ionization energy (IE) is the minimum amount of energy required to remove an electron from a gaseous atom in its ground electronic state. It quantifies the strength of the electrostatic attraction between the nucleus and its outermost electron. The higher the ionization energy, the more difficult it is to remove an electron, signifying a stronger hold on the electron by the atom.
Periodic Trends: A Guiding Map
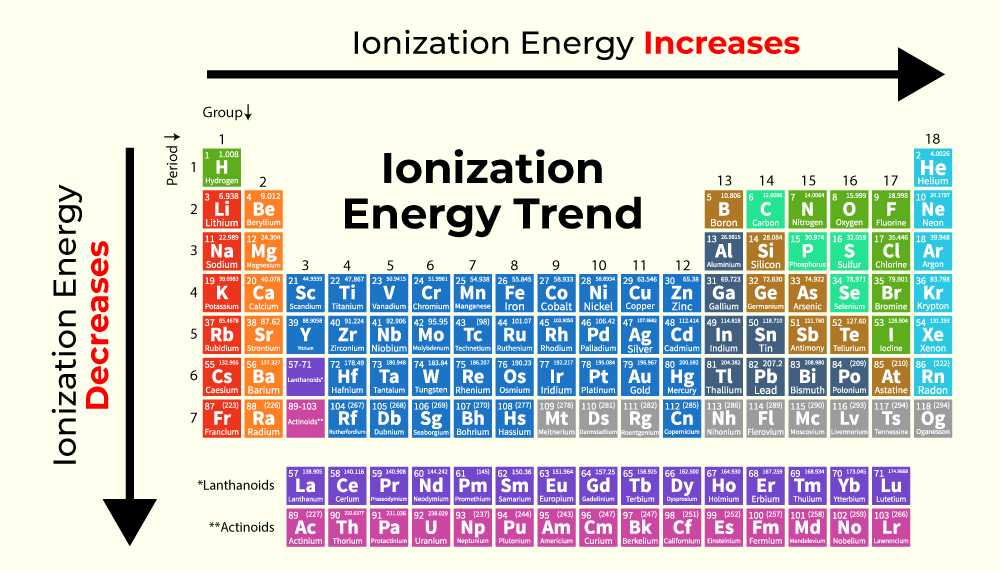
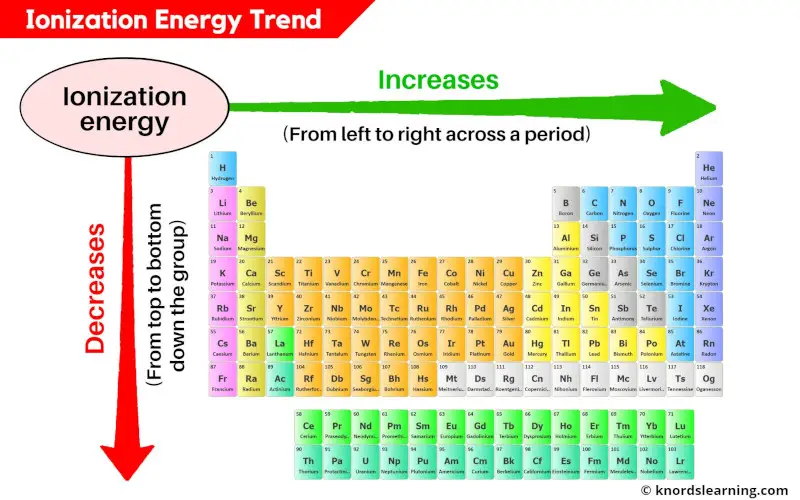
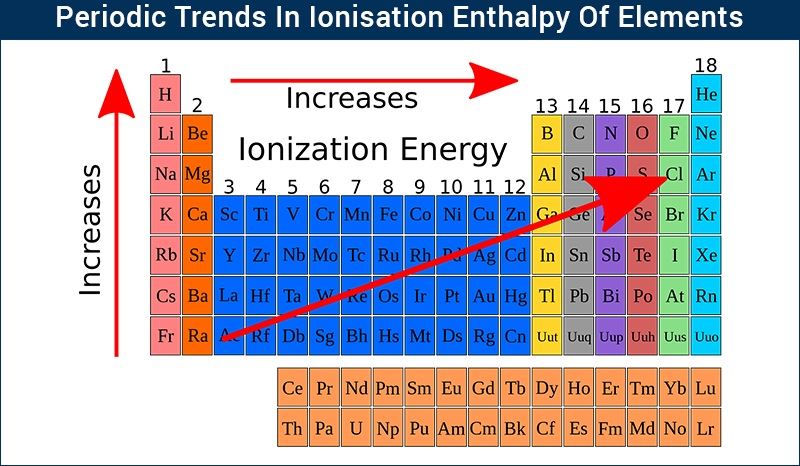
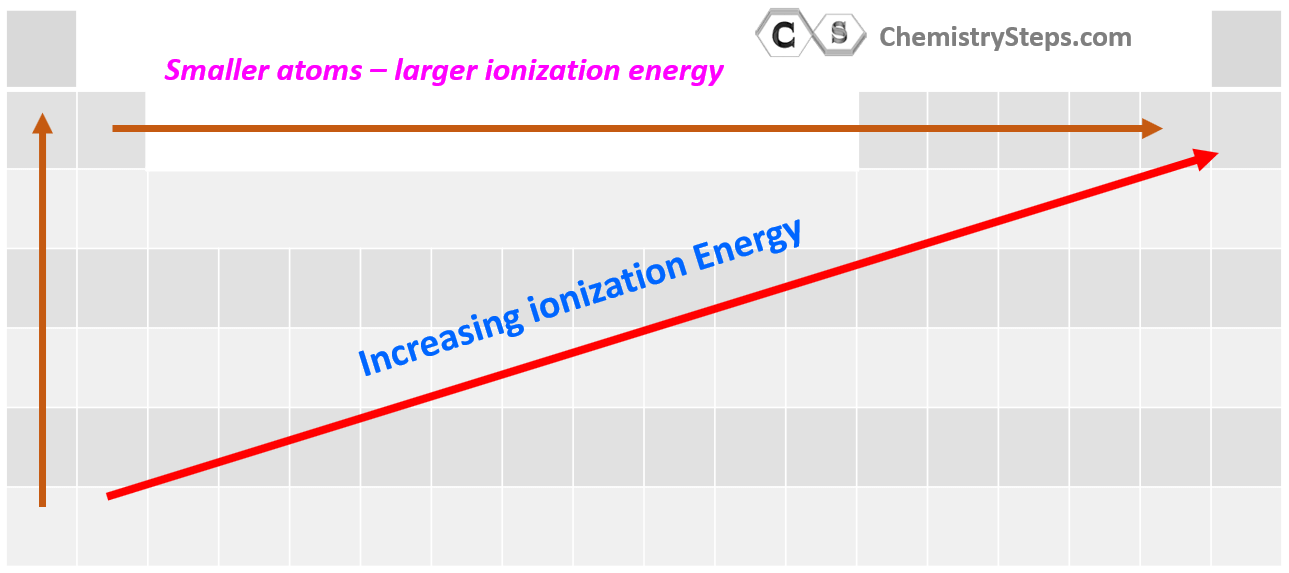
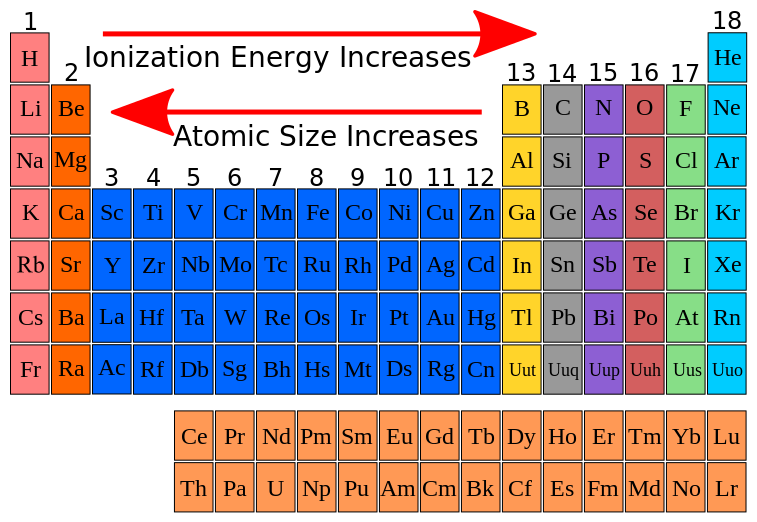
The periodic table reveals a systematic pattern in ionization energies, known as periodic trends. These trends provide a powerful tool for predicting the relative ease of ionization for different elements:
- Across a Period (Left to Right): Ionization energy generally increases as we move across a period from left to right. This is due to increasing nuclear charge, which pulls the electrons closer to the nucleus, strengthening the attraction and making it harder to remove an electron.
- Down a Group (Top to Bottom): Ionization energy generally decreases as we move down a group from top to bottom. This is attributed to the increasing atomic size. As the electron shells expand, the outermost electron is further from the nucleus, experiencing weaker attraction and becoming easier to remove.
Beyond the Basics: Factors Influencing Ionization Energy
While the general trends offer a valuable framework, several factors can influence ionization energy, leading to deviations from the expected pattern:
- Electron Configuration: The stability of electron configurations plays a significant role. Atoms with half-filled or completely filled subshells exhibit higher ionization energies due to their enhanced stability. For example, nitrogen (N) with its half-filled p-subshell has a higher IE than oxygen (O), which has a partially filled p-subshell.
- Shielding Effect: Inner electrons shield the outermost electron from the full nuclear charge. As the number of inner electrons increases, the shielding effect becomes more pronounced, weakening the attraction between the nucleus and the outermost electron, resulting in a lower ionization energy.
- Penetration Effect: The s-orbitals penetrate closer to the nucleus compared to p-orbitals. This penetration effect leads to a stronger attraction between the nucleus and s-electrons, resulting in higher ionization energies for elements with more s-electrons.
- Relativistic Effects: For heavier elements, relativistic effects become significant. These effects alter the energy levels of electrons, particularly those in the inner shells. The increased velocity of electrons near the nucleus leads to a contraction of the inner shells, resulting in a stronger attraction between the nucleus and the outer electrons, leading to higher ionization energies.
Applications: Unveiling the Potential
Ionization energy is not just a theoretical concept; it has practical implications in various fields:
- Spectroscopy: Analyzing the ionization energies of atoms and molecules provides valuable insights into their electronic structure and chemical bonding. Techniques like photoelectron spectroscopy rely on ionization energy measurements to study the energy levels of electrons in different states.
- Materials Science: Understanding ionization energy is crucial in designing materials with specific properties. For instance, elements with high ionization energies are often used in semiconductors, where their ability to hold onto electrons is essential for controlling electrical conductivity.
- Astrophysics: Ionization energies are instrumental in understanding the composition and evolution of stars. By analyzing the spectral lines emitted by stars, astronomers can determine the ionization states of different elements, providing clues about the temperature, pressure, and chemical composition of stellar atmospheres.
- Analytical Chemistry: Ionization energy is used in techniques like mass spectrometry to identify and quantify different compounds. By ionizing molecules and analyzing the masses of the resulting ions, scientists can determine the molecular structure and composition of complex mixtures.
Emerging Trends: Shaping the Future
The field of ionization energy is constantly evolving, driven by advancements in both experimental and theoretical approaches. Here are some notable trends shaping the future of this research:
- High-Precision Measurements: New experimental techniques, such as laser-based spectroscopy and ion trap methods, are enabling increasingly precise measurements of ionization energies. These advancements allow scientists to probe subtle effects and gain a deeper understanding of the factors influencing ionization.
- Theoretical Modeling: Sophisticated computational models are being developed to calculate ionization energies with high accuracy. These models incorporate relativistic effects and electron correlation, providing valuable insights into the electronic structure of atoms and molecules.
- Applications in Nanotechnology: Ionization energy plays a crucial role in developing novel nanomaterials with tailored properties. Understanding the ionization energies of nanoparticles and quantum dots enables the design of materials with specific optical, electrical, and catalytic properties.
- Beyond Single Ionization: Research is expanding beyond the first ionization energy to explore the energies required for removing multiple electrons. This knowledge is crucial for understanding the formation of highly charged ions, which are essential in fields like plasma physics and astrophysics.
Challenges and Opportunities:
Despite the remarkable progress in understanding ionization energy, several challenges remain:
- Accurate Predictions for Heavy Elements: Calculating accurate ionization energies for heavy elements is computationally demanding due to relativistic effects and electron correlation. Developing efficient and reliable computational methods for these elements is an ongoing challenge.
- Understanding Complex Systems: Predicting ionization energies in complex systems, such as molecules, clusters, and condensed phases, is a significant challenge. These systems exhibit intricate interactions between atoms and molecules, requiring advanced theoretical models and experimental techniques.
- Applications in Emerging Technologies: The potential applications of ionization energy are vast, but translating this knowledge into practical technologies requires further research and development. For example, harnessing ionization energy for energy storage and conversion technologies holds immense promise.
Conclusion: A Continued Journey of Discovery
Ionization energy, a fundamental property of atoms, continues to be a subject of intense research and exploration. As we move forward into 2025 and beyond, advancements in experimental techniques, theoretical modeling, and applications will further deepen our understanding of this crucial concept. From unraveling the mysteries of the cosmos to developing cutting-edge technologies, ionization energy will continue to play a vital role in shaping our scientific knowledge and technological advancements. The periodic journey through the elements, guided by the principles of ionization energy, promises to be an exciting and fruitful endeavor for years to come.


.PNG)