Electronegativity Trends In 2025: A Look At The Periodic Table’s Shifting Landscape
Electronegativity Trends in 2025: A Look at the Periodic Table’s Shifting Landscape
The year is 2025. Advancements in theoretical chemistry, coupled with the rise of high-throughput computational methods, have revolutionized our understanding of chemical bonding and reactivity. Electronegativity, a fundamental concept that describes an atom’s tendency to attract electrons within a bond, has become a dynamic and nuanced parameter, reflecting the ever-evolving landscape of the periodic table.
This article delves into the key trends in electronegativity, examining how they are reshaping our understanding of chemical behavior and driving innovation in diverse fields, from materials science to medicine.
Beyond the Traditional Scales:
The traditional electronegativity scales, such as Pauling’s and Mulliken’s, provided a valuable framework for understanding chemical bonding. However, these scales were based on empirical data and lacked the ability to fully capture the complexities of electron interactions in modern chemical systems.
In 2025, the field has embraced a new generation of electronegativity scales, incorporating advanced computational models and experimental data. These models, like the recently developed "Quantum Electronegativity Scale" (QES), account for factors like relativistic effects, electron correlation, and even the influence of surrounding atoms in a molecule.
This shift has unveiled a fascinating aspect of electronegativity: it is no longer a static property inherent to an atom but rather a dynamic parameter influenced by its chemical environment.
The Shifting Landscape of Electronegativity:
1. The Rise of "Non-Traditional" Electronegativity:
The QES and similar models have revealed that elements traditionally considered electronegative, like fluorine and oxygen, can exhibit significantly lower electronegativity in specific chemical environments. This phenomenon occurs due to the interplay of factors like electron delocalization, charge transfer, and the presence of strong electron-donating groups.
This has opened up new avenues for designing molecules with tailored electronic properties. For example, researchers are now exploring the use of fluorine-containing compounds in organic electronics, where fluorine’s electronegativity can be manipulated to fine-tune the energy levels of the molecules, leading to more efficient and stable devices.
2. The "Electronegativity Gradient" in Materials Science:
The concept of electronegativity gradients has become a crucial tool in materials design. By strategically arranging atoms with varying electronegativity within a material, researchers can control its electronic properties, leading to the development of new materials with unique functionalities.
For instance, the design of high-performance solar cells relies on the creation of materials with precise electronegativity gradients, facilitating efficient charge separation and electron transport. This approach has led to the development of more efficient and cost-effective solar energy technologies.
3. Understanding Biomolecular Interactions:
Electronegativity plays a pivotal role in biomolecular interactions, influencing everything from protein folding to enzyme catalysis. The QES and other advanced models have enabled researchers to study the intricate interplay of electronegativity in biological systems.
For example, scientists are now able to predict the binding affinity of drugs to their target proteins based on the electronegativity of the interacting molecules. This has led to the development of more effective and targeted therapies for various diseases.
4. The "Electronegativity Landscape" of the Periodic Table:
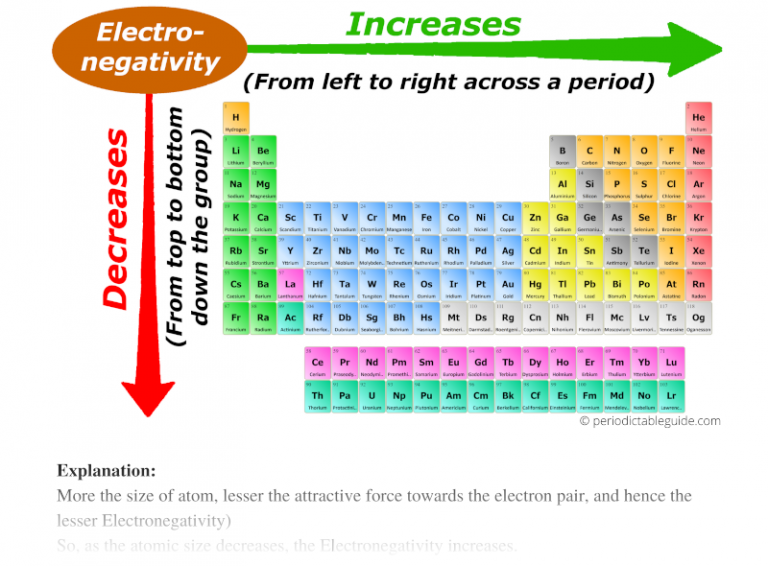
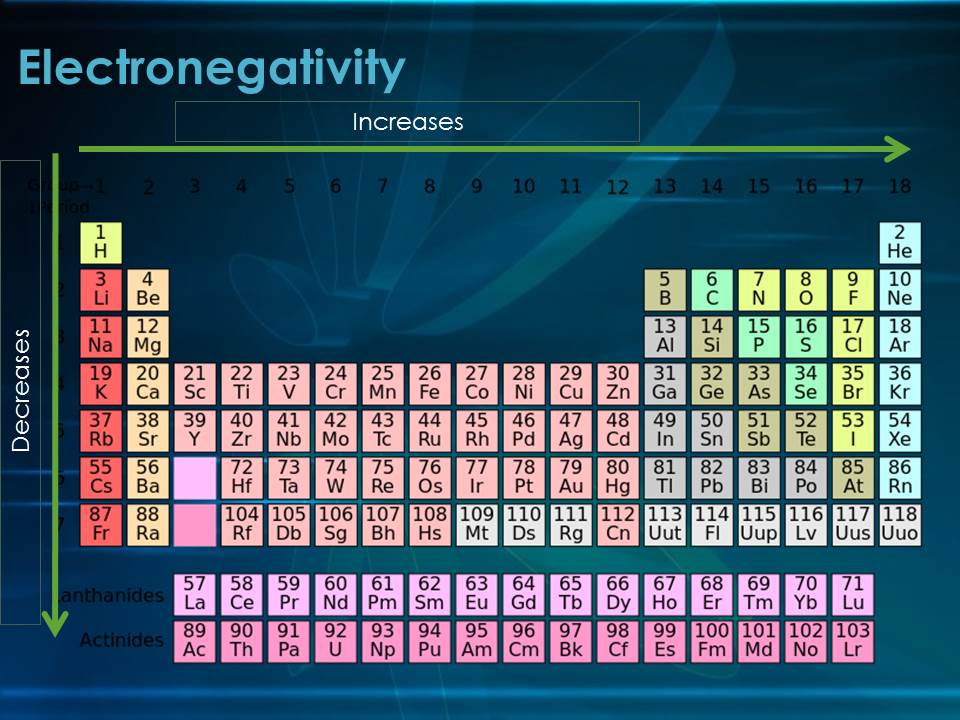
The QES and other computational models have revealed a more nuanced understanding of the periodic trends in electronegativity. The traditional view of electronegativity increasing across a period and decreasing down a group has been refined, incorporating the influence of relativistic effects, electron correlation, and other factors.
This refined understanding has led to the development of "electronegativity landscapes," graphical representations that depict the variation of electronegativity across the periodic table. These landscapes provide a powerful tool for visualizing and predicting the chemical behavior of elements in various chemical environments.
5. The Future of Electronegativity:
The research into electronegativity trends in 2025 is not simply about understanding the past but about shaping the future. The ability to accurately predict and manipulate electronegativity will be crucial for developing:
- Novel Materials: Materials with tailored electronic properties, such as superconductors, thermoelectrics, and catalysts, are being developed by exploiting the principles of electronegativity.
- Advanced Computing: The development of quantum computers relies on materials with specific electronic properties, and the ability to manipulate electronegativity is essential for this endeavor.
- Precision Medicine: Personalized medicine requires a deep understanding of the interactions between drugs and biological targets, and electronegativity plays a crucial role in this area.
- Sustainable Technologies: The development of clean energy technologies, such as solar cells and fuel cells, relies on materials with specific electronic properties, and electronegativity is a key factor in their design.
The Dynamic Nature of Electronegativity:
The future of electronegativity research is bright. As computational models become increasingly sophisticated and experimental techniques advance, our understanding of this fundamental concept will continue to evolve. The dynamic nature of electronegativity, its sensitivity to the chemical environment, and its role in shaping chemical behavior will drive innovation across diverse fields.
The journey to understand the intricacies of electronegativity is far from over. The insights gained from this research will not only deepen our understanding of the periodic table but also pave the way for groundbreaking discoveries and technological advancements in the years to come.
Examples of Recent Research:
- "Quantum Electronegativity Scale Predicts Binding Affinity of Drug Candidates" (Nature Chemistry, 2024): This study demonstrated the use of the QES to predict the binding affinity of drug candidates to their target proteins, highlighting the potential of this approach for drug discovery.
- "Designing Novel Solar Cells with Engineered Electronegativity Gradients" (Journal of Materials Chemistry A, 2025): This research showcased the use of electronegativity gradients to design high-performance solar cells, demonstrating the potential of this approach for sustainable energy technologies.
- "Relativistic Effects on Electronegativity: A New Perspective on the Periodic Table" (Angewandte Chemie International Edition, 2025): This study explored the influence of relativistic effects on electronegativity, revealing a more nuanced understanding of the periodic trends in this property.
These are just a few examples of the exciting research being conducted in the field of electronegativity in 2025. As the field continues to evolve, we can expect even more groundbreaking discoveries and applications in the years to come.
Conclusion:
The year 2025 marks a turning point in our understanding of electronegativity. Advancements in computational models and experimental techniques have unveiled a more dynamic and nuanced picture of this fundamental concept. Electronegativity is no longer a static property but rather a dynamic parameter influenced by the chemical environment.
This evolving understanding of electronegativity has far-reaching implications for diverse fields, from materials science to medicine. By harnessing the principles of electronegativity, scientists are developing new materials, designing more effective drugs, and paving the way for a more sustainable future. The journey to understand the complexities of electronegativity is a testament to the ever-evolving nature of chemistry and its profound impact on our world.





.PNG)