Electronegativity Trends In 2025: A Look At The Past, Present, And Future

Electronegativity Trends in 2025: A Look at the Past, Present, and Future
Electronegativity, the measure of an atom’s ability to attract electrons in a chemical bond, is a fundamental concept in chemistry. It dictates the nature of chemical bonds, the polarity of molecules, and ultimately, the properties and reactivity of countless chemical compounds. While the basic principles of electronegativity remain unchanged, the field is constantly evolving, fueled by advancements in computational chemistry, materials science, and our understanding of the interactions between atoms.
This article delves into the trends shaping the field of electronegativity in 2025, exploring the past, present, and future of this crucial concept. We’ll examine the role of electronegativity in the development of new materials, the challenges in accurately predicting electronegativity values, and the potential for new discoveries in the realm of chemical bonding.
A Brief History of Electronegativity
The concept of electronegativity was first introduced by Linus Pauling in 1932. Pauling recognized that the strength of a chemical bond was influenced by the relative attraction of the atoms involved. He proposed a scale, now known as the Pauling electronegativity scale, which assigns a numerical value to each element based on its ability to attract electrons.
Since Pauling’s groundbreaking work, numerous other electronegativity scales have been developed, each with its own strengths and limitations. These scales are based on various theoretical frameworks and experimental data, including:
- Mulliken Electronegativity: This scale, proposed by Robert Mulliken in 1934, defines electronegativity as the average of an atom’s ionization energy and electron affinity.
- Allred-Rochow Electronegativity: This scale, proposed by Allred and Rochow in 1958, utilizes the effective nuclear charge and the atomic radius to determine electronegativity.
- Sanderson Electronegativity: This scale, proposed by Sanderson in 1951, defines electronegativity as the resistance of an atom to the loss of electrons.
Each scale offers a different perspective on electronegativity, contributing to our understanding of its complexities. However, all scales share the fundamental principle of measuring an atom’s ability to attract electrons.
Electronegativity in the 21st Century: A Paradigm Shift
The field of electronegativity has undergone a significant transformation in the 21st century. The advent of powerful computers and advanced computational methods has revolutionized our ability to model and predict chemical behavior. This has led to a shift from purely empirical approaches to a more theoretical understanding of electronegativity.
Computational Chemistry and Electronegativity:
Computational chemistry has emerged as a powerful tool for studying electronegativity. Software programs, such as Gaussian and ORCA, enable researchers to calculate electronegativity values for various elements and molecules with unprecedented accuracy. This approach allows for the exploration of electronegativity in systems that are difficult or impossible to study experimentally.
Beyond the Periodic Trends:
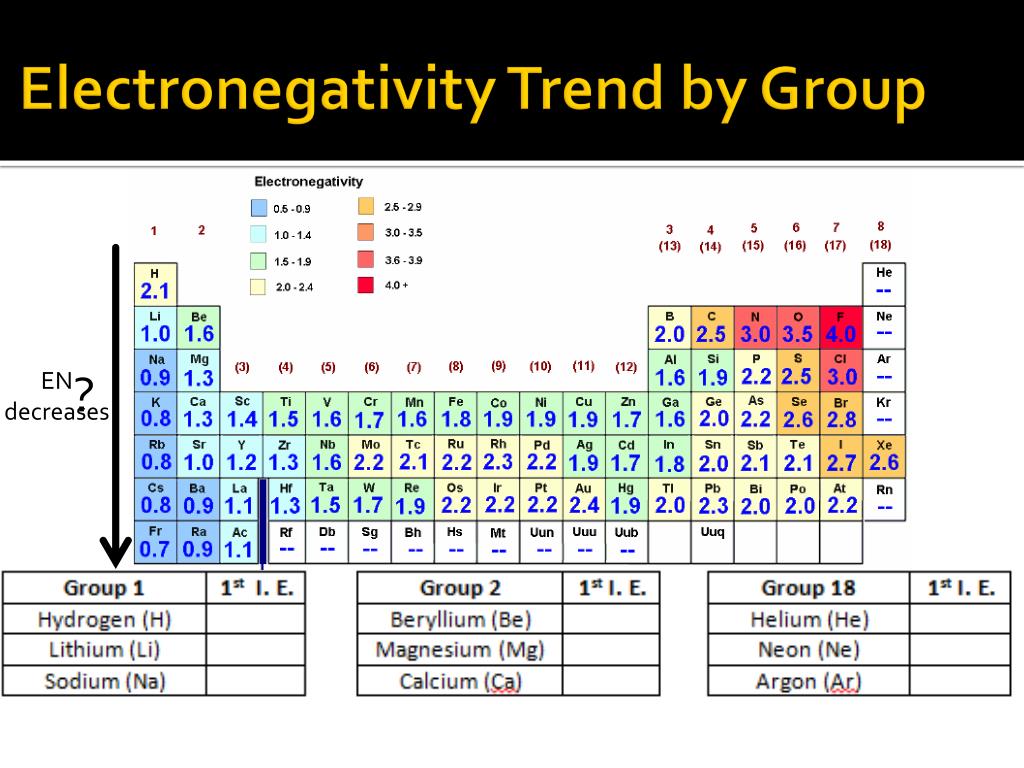
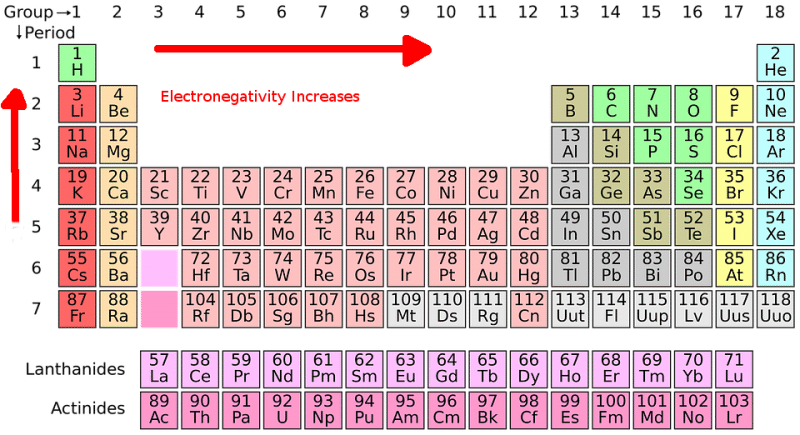
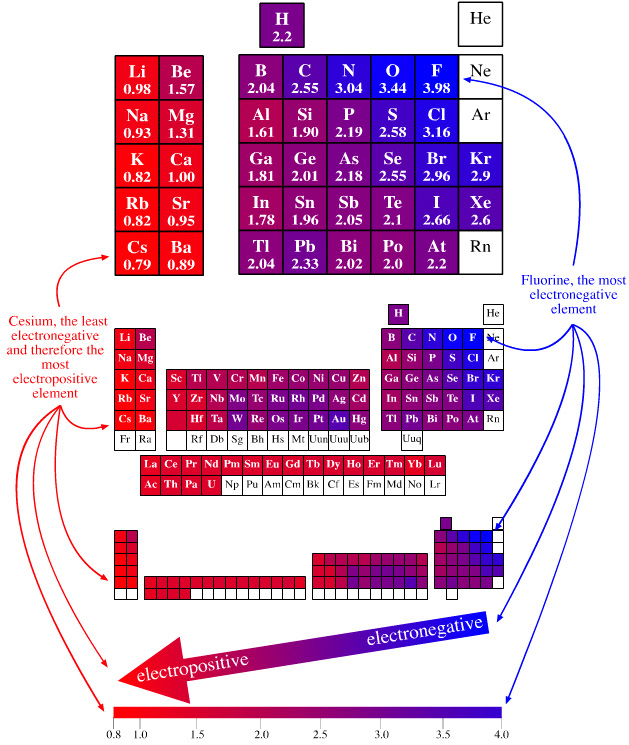
Traditionally, electronegativity was understood primarily through periodic trends. Elements on the right side of the periodic table, like oxygen and fluorine, tend to have higher electronegativity values due to their strong attraction for electrons. However, computational chemistry has revealed that electronegativity can be influenced by factors beyond the periodic table, such as:
- Coordination Environment: The number and type of atoms surrounding a central atom can significantly impact its electronegativity. This is particularly relevant in complex molecules and materials.
- Bond Order: The strength of a chemical bond, determined by its bond order, can also influence electronegativity. For example, a double bond will generally exhibit a higher electronegativity than a single bond.
- Electron Correlation: The interactions between electrons within a molecule can significantly impact the distribution of electron density and, consequently, electronegativity.
Challenges and Future Directions
Despite the advancements in computational chemistry, several challenges remain in accurately predicting and understanding electronegativity:
1. The Complexity of Electron Correlation: Accurate calculations of electronegativity require sophisticated methods for capturing electron correlation effects. These methods are computationally demanding, limiting their application to smaller molecules.
2. The Influence of Environment: Electronegativity can be significantly influenced by the surrounding environment, including the presence of other molecules, solvents, and even surfaces. Developing accurate models that account for these environmental effects is a major challenge.
3. The Need for Experimental Validation: While computational methods are powerful, they need to be validated against experimental data. Developing new experimental techniques for directly measuring electronegativity is crucial for refining our understanding of this complex concept.
Electronegativity in Materials Science
Electronegativity plays a critical role in materials science, influencing the properties and applications of diverse materials. Understanding the electronegativity of elements within a material allows scientists to predict its:
- Bonding Nature: The electronegativity difference between atoms determines the type of bond formed (ionic, covalent, or metallic).
- Polarity: The electronegativity difference within a molecule determines its polarity, influencing its solubility and reactivity.
- Electronic Conductivity: The electronegativity of elements can influence the electronic conductivity of a material, making it suitable for applications like semiconductors and conductors.
The Future of Electronegativity
The future of electronegativity research promises exciting discoveries and advancements in several areas:
- Predictive Materials Design: By leveraging computational chemistry, researchers can design new materials with specific properties based on their desired electronegativity characteristics. This will enable the development of novel materials for applications in energy storage, catalysis, and electronics.
- Understanding Biological Systems: Electronegativity plays a crucial role in biological systems, influencing the interactions between biomolecules, such as proteins and DNA. Further research into the role of electronegativity in biological systems could lead to breakthroughs in drug discovery and disease treatment.
- Developing New Scales: The current electronegativity scales may not adequately capture the complexities of electronegativity in all systems. Developing new scales that account for factors like coordination environment and bond order will be essential for a more comprehensive understanding of electronegativity.
Conclusion
Electronegativity remains a fundamental concept in chemistry, influencing the properties and reactivity of countless chemical compounds. While the basic principles of electronegativity have remained unchanged, the field is constantly evolving, driven by advancements in computational chemistry, materials science, and our understanding of the interactions between atoms.
The future of electronegativity research promises exciting advancements in predicting materials properties, understanding biological systems, and developing new scales that better capture the complexities of this crucial concept. As we continue to explore the intricacies of electronegativity, we unlock new possibilities for the development of innovative materials, technologies, and solutions to global challenges.