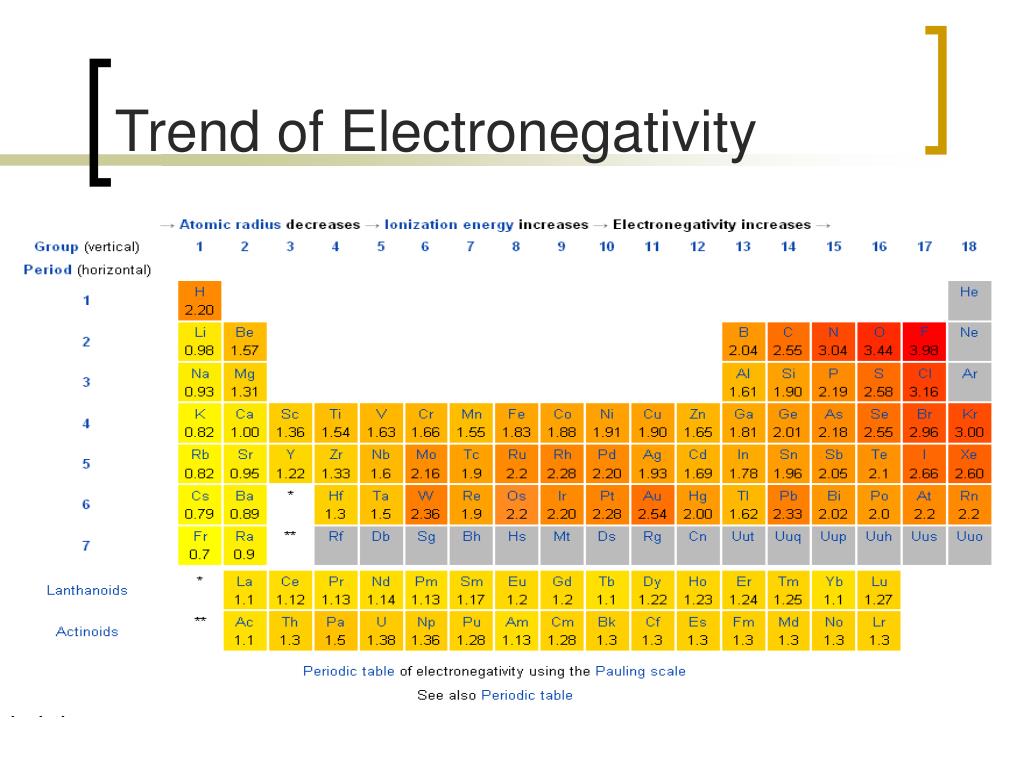
Electronegativity Trends In 2025: A Journey Towards Predictive Power
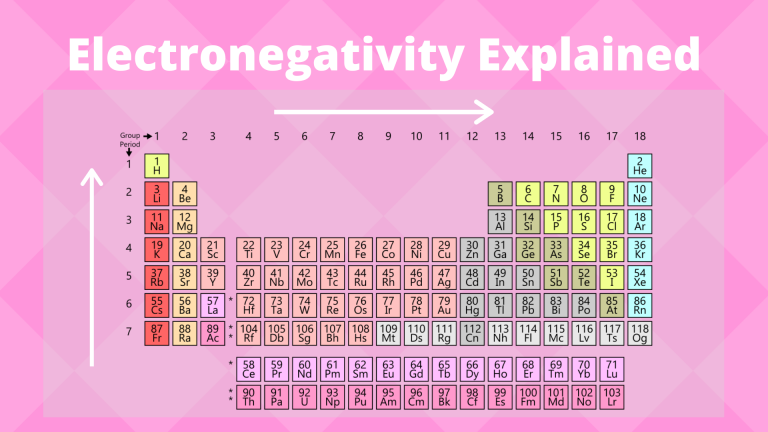
Electronegativity Trends in 2025: A Journey Towards Predictive Power
Electronegativity, a fundamental concept in chemistry, quantifies an atom’s tendency to attract electrons within a chemical bond. Its importance extends far beyond textbook definitions, influencing molecular properties, reactivity, and even the design of novel materials. As we approach 2025, the field of electronegativity is experiencing a renaissance, fueled by advancements in computational chemistry, experimental techniques, and an increasing desire to understand and predict chemical behavior.
The Shifting Landscape of Electronegativity:
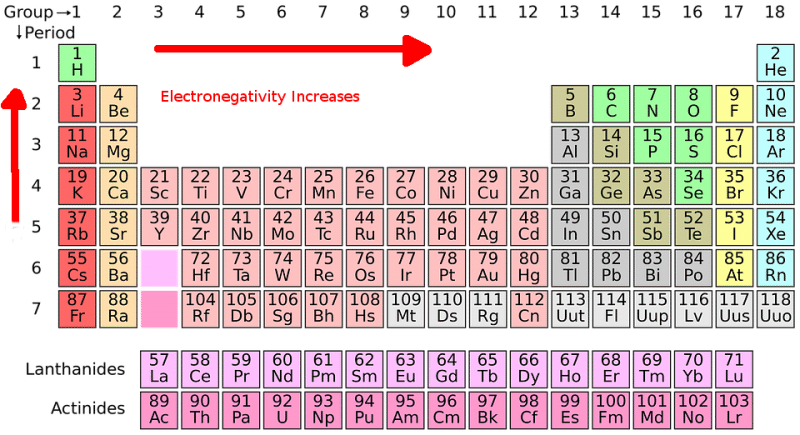
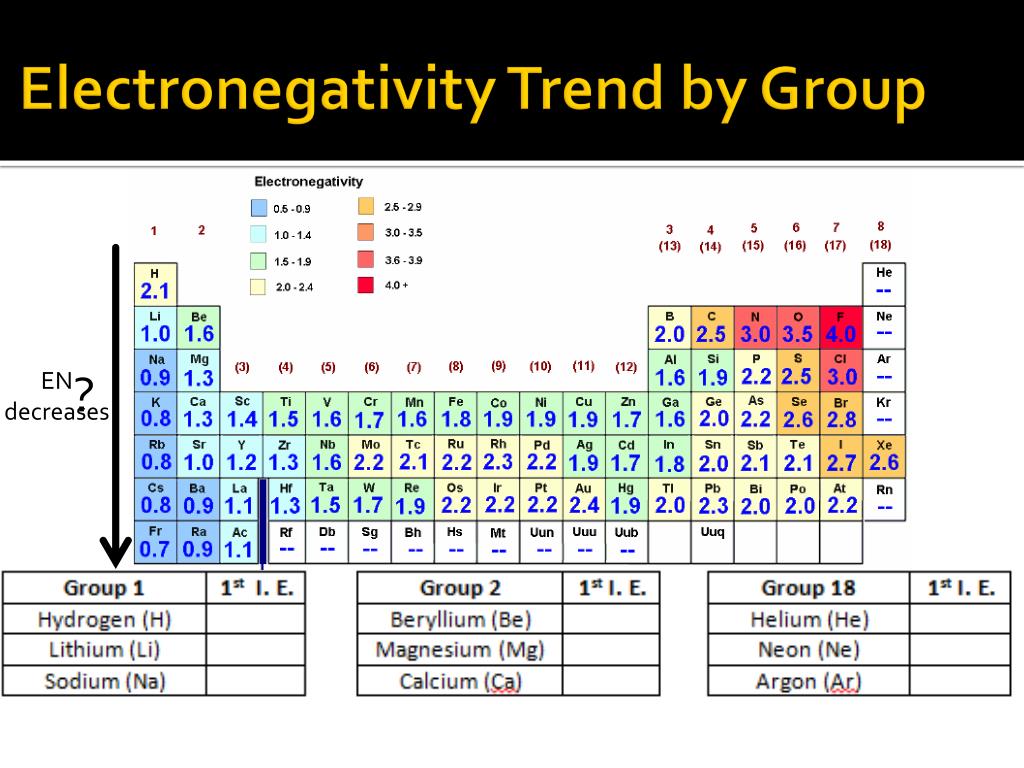
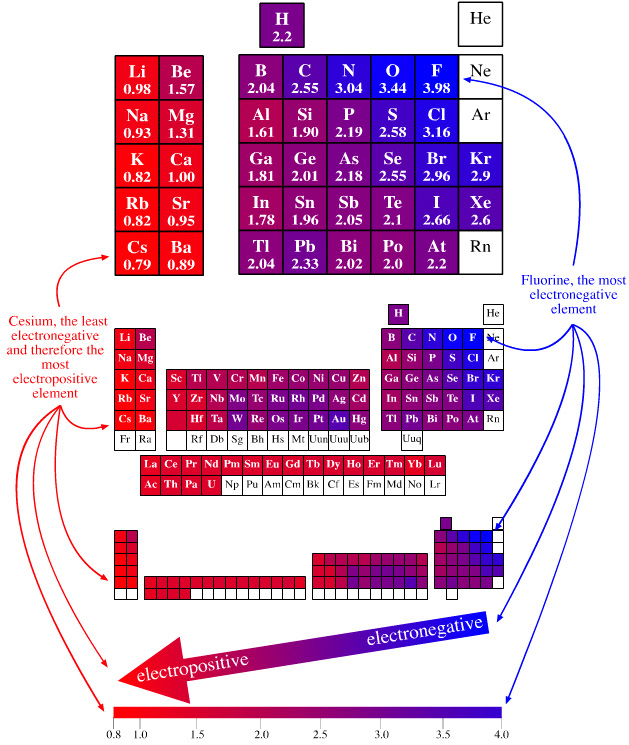
The traditional understanding of electronegativity, often represented by the Pauling scale, has served chemists well for decades. However, its limitations in capturing the nuances of complex chemical systems have become increasingly apparent. This has led to the development of several alternative electronegativity scales, each with its own strengths and weaknesses.
1. Beyond Pauling: A Multifaceted Approach:
- Mulliken Electronegativity: This scale, based on ionization energy and electron affinity, offers a more theoretical perspective, emphasizing the energetic basis of electronegativity.
- Allen Electronegativity: This scale, derived from atomic orbital energies, provides insights into the influence of atomic structure on electronegativity.
- Sanderson Electronegativity: This scale, based on the concept of electron density, highlights the importance of electron distribution in determining electronegativity.
These alternative scales, alongside computational methods like Density Functional Theory (DFT), offer a more nuanced understanding of electronegativity, accounting for factors like bond type, coordination environment, and even the influence of surrounding atoms.
2. Unveiling the Dynamics of Electronegativity:
Research in 2025 will focus on understanding the dynamic nature of electronegativity. This involves investigating how electronegativity changes in response to:
- External Stimuli: External factors like pressure, temperature, and electric fields can significantly influence the electron distribution within molecules, leading to dynamic changes in electronegativity.
- Chemical Environment: The presence of neighboring atoms and functional groups can significantly modify the electronegativity of an atom, creating complex local electronegativity landscapes.
- Bond Formation: The formation of chemical bonds itself can alter the electronegativity of participating atoms, influencing the strength and polarity of the bond.
By understanding these dynamic aspects of electronegativity, researchers aim to develop predictive models for chemical behavior, enabling a deeper understanding of reactivity, bond formation, and molecular properties.
3. The Rise of Computational Electronegativity:
The advancement of computational chemistry has revolutionized the study of electronegativity. DFT and other quantum chemical methods allow researchers to calculate electronegativity with unprecedented accuracy, providing insights into:
- Theoretical Electronegativity: Computational approaches enable the calculation of electronegativity for hypothetical molecules and exotic chemical environments, expanding the scope of our understanding.
- Electronegativity Maps: These maps, generated through computational modeling, depict the spatial distribution of electronegativity within molecules, providing a visual representation of electron density and reactivity.
- Predictive Power: Computational models can be used to predict the electronegativity of novel materials, enabling the design of compounds with specific properties, like enhanced reactivity or increased stability.
4. Experimental Validation: Bridging the Gap:
Computational models are powerful tools, but their predictions need experimental validation. In 2025, experimental techniques will play a crucial role in:
- Spectroscopic Methods: Techniques like X-ray photoelectron spectroscopy (XPS) and Nuclear Magnetic Resonance (NMR) provide direct experimental evidence for the electron distribution within molecules, allowing for the validation of computational predictions.
- Electrochemical Measurements: Techniques like cyclic voltammetry can be used to measure the reduction potential of molecules, providing insights into their electron affinity and, indirectly, their electronegativity.
- Microscopy Techniques: Advanced microscopy techniques, like scanning tunneling microscopy (STM) and atomic force microscopy (AFM), allow for the visualization of electron density at the atomic scale, providing experimental validation of computational electronegativity maps.
By combining computational and experimental approaches, researchers aim to develop a comprehensive understanding of electronegativity, bridging the gap between theoretical predictions and experimental observations.
Applications of Electronegativity in 2025:
The advancements in understanding and predicting electronegativity will have profound implications for various fields:
1. Materials Science:
- Design of Novel Materials: By understanding the influence of electronegativity on material properties, researchers can design materials with specific characteristics, like enhanced conductivity, catalytic activity, or optical properties.
- Predicting Stability and Reactivity: Electronegativity plays a crucial role in determining the stability and reactivity of materials, enabling the design of materials resistant to degradation or tailored for specific chemical reactions.
2. Drug Discovery and Development:
- Predicting Drug-Target Interactions: Electronegativity is a key factor in determining the strength and specificity of drug-target interactions, enabling the design of drugs with improved efficacy and reduced side effects.
- Understanding Drug Metabolism: Electronegativity influences the metabolism of drugs within the body, enabling the prediction of drug clearance and the design of drugs with optimized pharmacokinetic properties.
3. Environmental Science:
- Understanding Environmental Chemistry: Electronegativity plays a crucial role in understanding the fate and transport of pollutants in the environment, enabling the development of strategies for environmental remediation.
- Designing Sustainable Materials: By understanding the influence of electronegativity on material stability and reactivity, researchers can design sustainable materials that are less prone to degradation and minimize environmental impact.
4. Nanotechnology:
- Designing Nanomaterials: Electronegativity is essential for designing nanomaterials with specific properties, like enhanced conductivity, catalytic activity, or optical properties.
- Understanding Nanoscale Interactions: Electronegativity plays a critical role in understanding the interactions between nanoparticles and their environment, enabling the design of nanoscale devices with specific functionalities.
Challenges and Future Directions:
Despite the significant progress in understanding electronegativity, several challenges remain:
- Developing More Accurate Computational Models: Computational models need further refinement to accurately capture the complex interactions and dynamic nature of electronegativity in diverse chemical environments.
- Bridging the Gap Between Theory and Experiment: Continued efforts are needed to develop experimental techniques that can directly measure electronegativity with high precision and accuracy, allowing for robust validation of computational predictions.
- Understanding the Role of Electronegativity in Complex Systems: Further research is needed to understand the role of electronegativity in complex systems, like biological molecules and materials with multiple components, where the interplay of different electronegativity values can lead to emergent properties.
Looking Ahead:
The future of electronegativity research holds exciting possibilities. As computational models become more sophisticated and experimental techniques continue to advance, we can expect a deeper understanding of this fundamental concept. This knowledge will enable us to design novel materials with tailored properties, develop more effective drugs, and address pressing environmental challenges. The journey towards a predictive understanding of electronegativity is ongoing, promising a future where chemistry is not just about understanding the past, but also about predicting the future.
Conclusion:
Electronegativity, once a static concept, is evolving into a dynamic and multifaceted tool for understanding and predicting chemical behavior. The advancements in computational chemistry, experimental techniques, and the growing need for predictive power in diverse fields are driving this renaissance. As we move towards 2025, the field of electronegativity is poised to play an increasingly pivotal role in shaping the future of materials science, drug discovery, environmental science, and nanotechnology. The journey towards a deeper understanding of electronegativity is not just about unraveling the secrets of the past, but about unlocking the potential of the future.