Electronegativity In 2025: A Look At The Trends Shaping Chemical Bonding
Electronegativity in 2025: A Look at the Trends Shaping Chemical Bonding
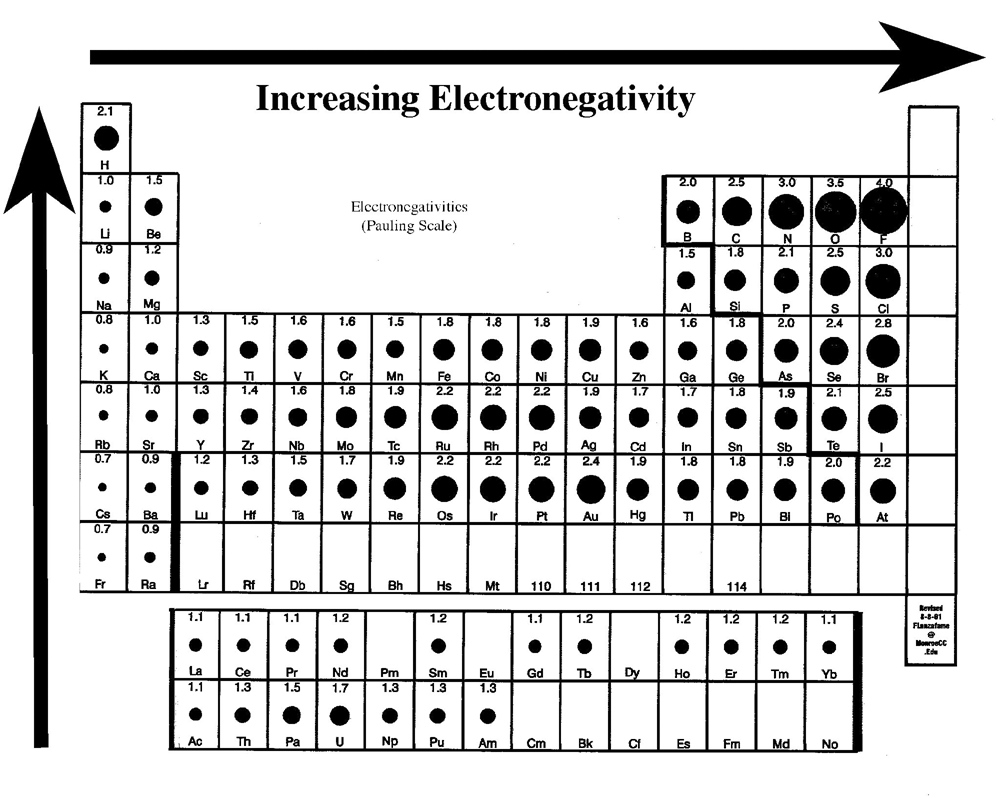
Electronegativity, the measure of an atom’s ability to attract electrons in a chemical bond, is a fundamental concept in chemistry. It governs the nature of chemical bonds, the polarity of molecules, and ultimately, the properties and reactivity of compounds. As we approach 2025, advancements in computational chemistry, material science, and nanotechnology are ushering in a new era of understanding and manipulating electronegativity, leading to exciting trends with significant implications for various fields.
The Rise of Computational Chemistry:
Computational chemistry has revolutionized our understanding of electronegativity. Sophisticated quantum chemical methods, coupled with ever-increasing computational power, allow us to calculate electronegativity values with unprecedented accuracy. This has led to the development of new electronegativity scales, including the recently proposed "Dynamic Electronegativity" scale that accounts for the changing electron density within a molecule during bond formation.
Beyond Traditional Electronegativity:
While the traditional Pauling scale remains a valuable tool, the limitations of its static nature are becoming increasingly apparent. The concept of "dynamic electronegativity" acknowledges that an atom’s electronegativity is not constant but varies depending on its chemical environment. This dynamic perspective is crucial for understanding complex chemical reactions, especially those involving transition metals and organometallic compounds.
Electronegativity in Materials Science:
The ability to fine-tune electronegativity is becoming a powerful tool in materials science. By strategically combining elements with varying electronegativities, researchers are able to design materials with specific properties. For example, manipulating electronegativity in semiconductors can lead to the development of more efficient solar cells and transistors. Similarly, by controlling the electronegativity of metal oxides, we can create new catalysts with enhanced activity and selectivity.
Nanotechnology and Electronegativity:
The realm of nanotechnology offers a unique opportunity to explore the influence of electronegativity on a smaller scale. At the nanoscale, the surface-to-volume ratio increases dramatically, making surface properties, including electronegativity, critically important. This has led to the development of new materials like nanowires and quantum dots with tunable electronic properties based on their surface electronegativity.
Electronegativity in Biology and Medicine:
Electronegativity plays a crucial role in biological systems, influencing protein folding, enzyme activity, and drug-receptor interactions. Understanding and manipulating electronegativity in biological systems holds immense promise for developing new drugs, targeting specific proteins, and understanding disease mechanisms.
Key Trends Shaping Electronegativity in 2025:
-
Personalized Medicine: By analyzing the electronegativity of specific proteins and enzymes within an individual, personalized medicine can tailor drug treatments for optimal efficacy and minimize side effects.
-
Advanced Materials Design: Computational methods will enable the precise prediction and design of materials with specific properties by strategically manipulating electronegativity. This will lead to the development of novel catalysts, high-performance batteries, and advanced electronic devices.
-
Nanomaterials with Tunable Properties: The ability to control electronegativity at the nanoscale will allow for the creation of nanomaterials with tailored optical, electrical, and magnetic properties, enabling the development of next-generation sensors, energy storage devices, and quantum computers.
-
Understanding Complex Chemical Reactions: Dynamic electronegativity models will be crucial for understanding and predicting the behavior of complex chemical reactions, particularly in fields like catalysis, organic chemistry, and materials science.
-
Beyond the Periodic Table: The exploration of electronegativity in unconventional systems like ionic liquids, metal-organic frameworks, and even exotic materials like graphene will expand our understanding of chemical bonding beyond traditional concepts.
Challenges and Opportunities:
Despite the remarkable progress in understanding and manipulating electronegativity, several challenges remain. Accurately predicting dynamic electronegativity in complex systems remains a significant challenge. Furthermore, developing experimental techniques to directly measure dynamic electronegativity in real-time is crucial for validating computational models and guiding material design.
The Future of Electronegativity:
The future of electronegativity research is filled with exciting possibilities. Continued advancements in computational chemistry, materials science, and nanotechnology will enable us to exploit the power of electronegativity to address global challenges in energy, health, and technology. As we delve deeper into the intricacies of chemical bonding, electronegativity will continue to be a cornerstone of our understanding of the molecular world, shaping the future of chemistry and beyond.
Specific Applications and Examples:
1. Catalysis:
- Designing Catalysts for CO2 Conversion: By manipulating the electronegativity of metal oxides, researchers can create catalysts with enhanced activity and selectivity for converting CO2 into valuable fuels and chemicals.
- Enhancing Enzyme Activity: Understanding the electronegativity of active sites in enzymes can help researchers design inhibitors or activators to modulate their activity for therapeutic purposes.
2. Energy Storage:
- Developing High-Capacity Batteries: By strategically combining elements with varying electronegativities, researchers can design battery electrodes with improved energy density and cycle life.
- Improving Solar Cell Efficiency: Manipulating the electronegativity of semiconductors can lead to more efficient charge separation and transport in solar cells, ultimately increasing their energy conversion efficiency.
3. Nanotechnology:
- Creating Tunable Nanowires: By controlling the electronegativity of the surface atoms, researchers can design nanowires with specific electronic properties for use in electronic devices and sensors.
- Developing Targeted Drug Delivery Systems: Understanding the electronegativity of drug molecules and target proteins can help design nanocarriers that specifically deliver drugs to diseased cells, minimizing side effects.
4. Biology and Medicine:
- Designing Novel Antibiotics: By targeting the electronegativity of specific bacterial proteins, researchers can develop new antibiotics that are effective against drug-resistant bacteria.
- Understanding Protein Folding: The electronegativity of amino acids plays a crucial role in protein folding and stability, influencing their function and susceptibility to disease.
Conclusion:
Electronegativity, a fundamental concept in chemistry, is undergoing a renaissance. Advancements in computational chemistry, materials science, and nanotechnology are pushing the boundaries of our understanding and manipulation of electronegativity. As we move forward, we can expect to see exciting applications of electronegativity in various fields, from personalized medicine and advanced materials design to the development of next-generation energy technologies and the exploration of the nanoscale world. The future of electronegativity research holds immense potential to revolutionize our world and address some of the most pressing challenges facing humanity.







