Electronegativity: A Timeless Trend In The Periodic Table (2025)
Electronegativity: A Timeless Trend in the Periodic Table (2025)
The periodic table, a cornerstone of chemistry, provides a framework for understanding the behavior of elements and their interactions. Among the fundamental properties that govern these interactions, electronegativity stands out as a key determinant of chemical bonding and reactivity. As we delve deeper into the intricacies of chemical behavior, the importance of electronegativity continues to grow, influencing our understanding of everything from the formation of molecules to the design of novel materials.
Electronegativity: A Fundamental Property
Electronegativity, a measure of an atom’s ability to attract electrons in a chemical bond, is a fundamental concept in chemistry. It plays a pivotal role in determining the nature of chemical bonds, influencing the polarity of molecules, and driving a myriad of chemical reactions.
Periodic Trends in Electronegativity
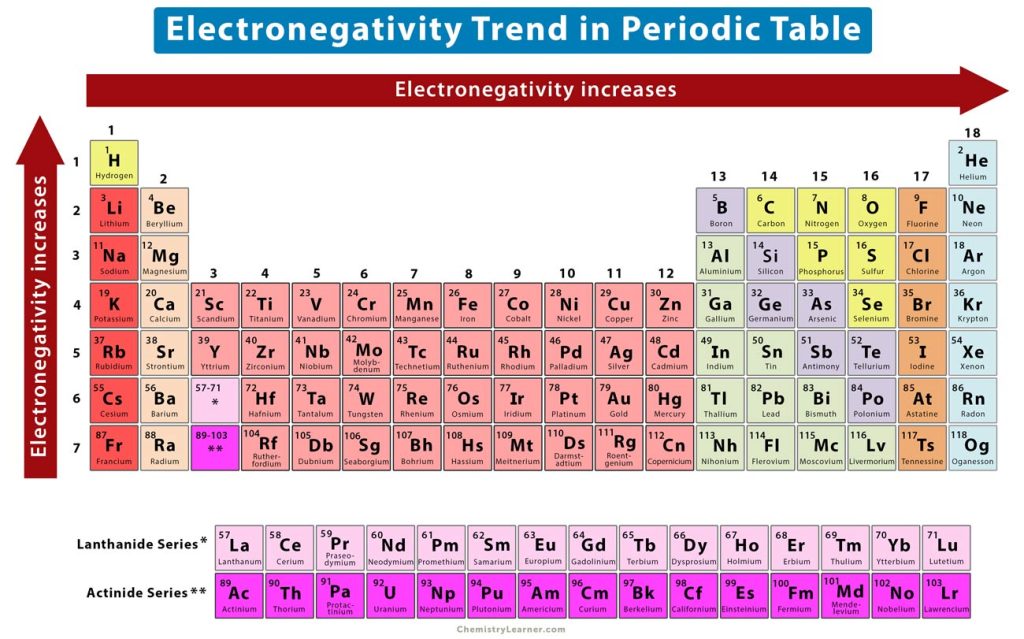
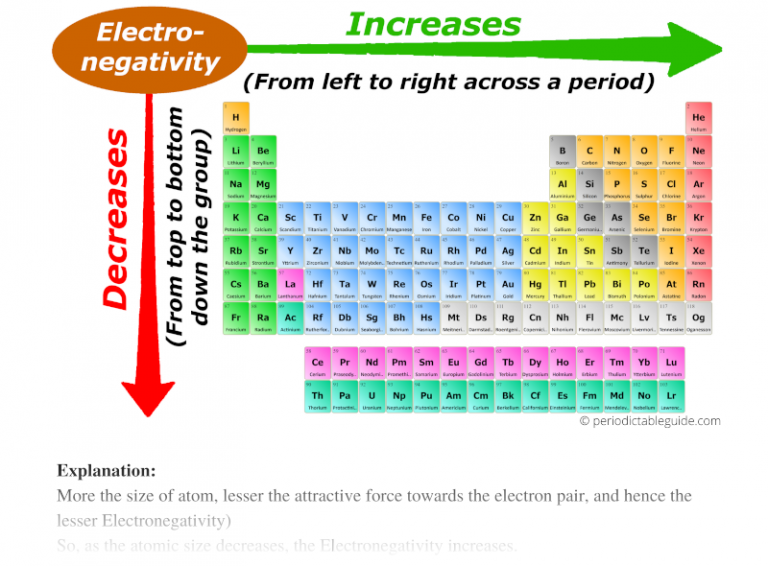
One of the most striking features of the periodic table is the systematic variation of electronegativity across periods and groups. Understanding these trends is crucial for predicting the behavior of elements and the nature of their compounds.
- Across a Period: Electronegativity generally increases from left to right across a period. This trend arises from the increasing nuclear charge experienced by valence electrons, leading to a stronger attraction towards the nucleus and a higher tendency to attract electrons in a bond.
- Down a Group: Electronegativity generally decreases down a group. This is attributed to the increasing distance between the nucleus and valence electrons, weakening the attraction between them. The shielding effect of inner electrons also plays a role, further reducing the effective nuclear charge experienced by valence electrons.
The Pauling Scale: A Universal Measure
The Pauling scale, developed by Linus Pauling in 1932, remains the most widely used method for quantifying electronegativity. This scale assigns numerical values to elements based on their ability to attract electrons in a covalent bond. Fluorine, the most electronegative element, is assigned a value of 4.0, while other elements are assigned values relative to fluorine.
Beyond the Pauling Scale: A Spectrum of Approaches
While the Pauling scale provides a valuable framework for understanding electronegativity, it is not without its limitations. Alternative scales, such as the Mulliken scale and the Allred-Rochow scale, offer different perspectives on electronegativity. These scales, while based on different theoretical frameworks, generally correlate well with the Pauling scale and provide insights into the nuances of electronegativity.
The Role of Electronegativity in Chemical Bonding
Electronegativity is a key factor in determining the type of chemical bond formed between two atoms.
- Covalent Bonding: When two atoms with similar electronegativities share electrons, a covalent bond is formed. The shared electrons are distributed equally between the atoms, resulting in a nonpolar covalent bond. However, if the electronegativity difference between the atoms is significant, the electrons are unevenly distributed, leading to a polar covalent bond.
- Ionic Bonding: When the electronegativity difference between two atoms is large, one atom completely transfers its electron to the other, resulting in the formation of ions. The electrostatic attraction between the oppositely charged ions forms an ionic bond.
Electronegativity and Chemical Reactions
Electronegativity plays a crucial role in determining the reactivity of elements and the course of chemical reactions.
- Oxidation and Reduction: Electronegativity is directly related to the tendency of an element to gain or lose electrons. Elements with high electronegativity are more likely to gain electrons and undergo reduction, while those with low electronegativity are more likely to lose electrons and undergo oxidation.
- Acid-Base Reactions: The electronegativity of an element can influence its acidic or basic properties. For instance, elements with high electronegativity tend to form strong acids, while those with low electronegativity tend to form strong bases.
- Catalysis: The electronegativity of a catalyst can influence its ability to promote a specific reaction. Catalysts with high electronegativity can attract electrons from reactants, facilitating bond breaking and formation.
Applications of Electronegativity in Modern Chemistry
The concept of electronegativity has found wide-ranging applications in modern chemistry, influencing our understanding of a diverse array of phenomena.
- Materials Science: Understanding electronegativity is crucial for designing materials with specific properties. For instance, the electronegativity of constituent elements can be manipulated to create materials with desired electrical conductivity, optical properties, or mechanical strength.
- Drug Design: Electronegativity plays a crucial role in drug design, influencing the interactions between drugs and target molecules. By understanding the electronegativity of drug molecules and their target sites, researchers can design drugs with enhanced binding affinity and therapeutic efficacy.
- Environmental Chemistry: Electronegativity is relevant to understanding environmental processes, such as the fate of pollutants in the environment. The electronegativity of pollutants can influence their reactivity and persistence in the environment, impacting their potential for bioaccumulation and toxicity.
Looking Ahead: The Future of Electronegativity
As our understanding of chemistry continues to evolve, the importance of electronegativity is likely to grow. Advancements in computational chemistry and theoretical modeling are providing new insights into the nuances of electronegativity and its role in chemical bonding and reactivity. These advancements are paving the way for a deeper understanding of complex chemical phenomena, leading to the development of novel materials and technologies.
Electronegativity: A Cornerstone of Chemical Understanding
Electronegativity, a fundamental property of atoms, plays a crucial role in shaping the chemical world. From the formation of bonds to the course of chemical reactions, electronegativity governs the interactions between atoms and molecules, influencing the properties of matter and the processes that drive life. As we continue to explore the intricacies of chemistry, electronegativity will remain a cornerstone of our understanding, guiding our quest to unravel the mysteries of the chemical universe.
Beyond the Basics: Exploring the Nuances of Electronegativity
While the basic trends in electronegativity are well-established, there are nuances that contribute to the complexity of this property.
- Orbital Hybridization: The hybridization of atomic orbitals can influence the electronegativity of an element. For instance, sp3 hybridized orbitals are generally less electronegative than sp2 hybridized orbitals.
- Electron Correlation Effects: Electron correlation effects, which account for the interactions between electrons in an atom, can also influence electronegativity. These effects are particularly significant for elements with multiple valence electrons.
- Relativistic Effects: For heavy elements, relativistic effects become increasingly important. These effects arise from the high speeds of electrons in heavy atoms and can influence the electronegativity of these elements.
Electronegativity: A Dynamic Property
Electronegativity is not a static property but rather a dynamic one that can vary depending on the chemical environment.
- Charge Density: The charge density of an atom can influence its electronegativity. For instance, a positively charged atom will be more electronegative than a neutral atom.
- Bonding Environment: The nature of the chemical bond can also affect electronegativity. For example, an atom in a polar covalent bond will be more electronegative than the same atom in a nonpolar covalent bond.
Challenges and Opportunities
While our understanding of electronegativity has advanced significantly, there are still challenges and opportunities for further research.
- Accurate Prediction of Electronegativity: Developing accurate methods for predicting electronegativity for different elements and in various chemical environments remains a challenge.
- Understanding Electronegativity in Complex Systems: Exploring the role of electronegativity in complex systems, such as biological systems and materials with multiple components, is an ongoing area of research.
- Developing Novel Applications: Applying the principles of electronegativity to develop new materials, technologies, and solutions for pressing global challenges is a key area of focus for future research.
Electronegativity: A Timeless Trend
The periodic trends in electronegativity, first observed by Linus Pauling nearly a century ago, continue to hold relevance in the modern era. As we delve deeper into the intricacies of chemical behavior, the importance of electronegativity is only growing. This fundamental property provides a powerful tool for understanding chemical bonding, predicting reactivity, and designing new materials and technologies. As we navigate the ever-evolving landscape of chemistry, electronegativity will remain a timeless trend, guiding our exploration of the chemical universe.




.PNG)

.PNG)