Electron Affinity Trends In 2025: A Glimpse Into The Future Of Chemical Bonding

Electron Affinity Trends in 2025: A Glimpse into the Future of Chemical Bonding
The year 2025 marks a pivotal moment in our understanding of electron affinity, a fundamental property that governs chemical bonding and reactivity. While the general trends are well-established, the relentless pursuit of knowledge in chemistry and materials science has unveiled new insights and complexities, pushing the boundaries of our understanding. This article delves into the evolving landscape of electron affinity trends, exploring key developments and their implications for the future of chemistry.
Electron Affinity: A Fundamental Concept
Electron affinity (EA) refers to the energy change that occurs when an atom in the gaseous state gains an electron to form a negative ion. It is a crucial parameter that dictates the tendency of an atom to accept electrons and participate in chemical reactions. A higher EA value indicates a greater tendency for an atom to form an anion.
Historical Trends and the Periodic Table
The periodic table serves as a powerful tool for predicting electron affinity trends. Historically, the following observations have been made:
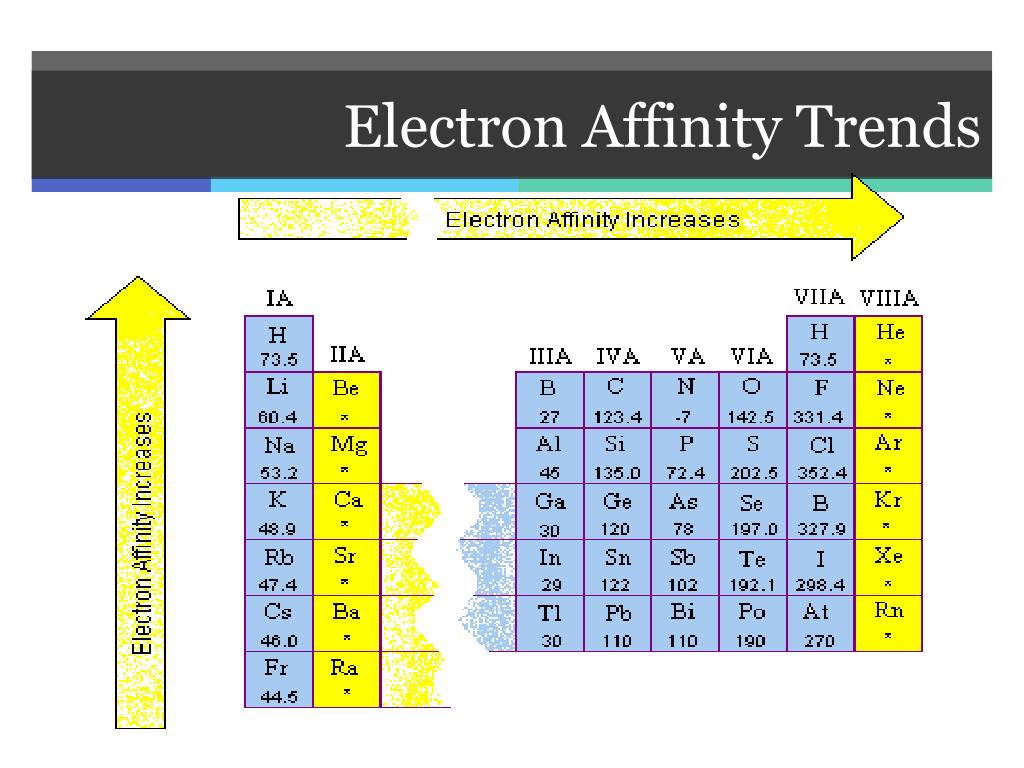
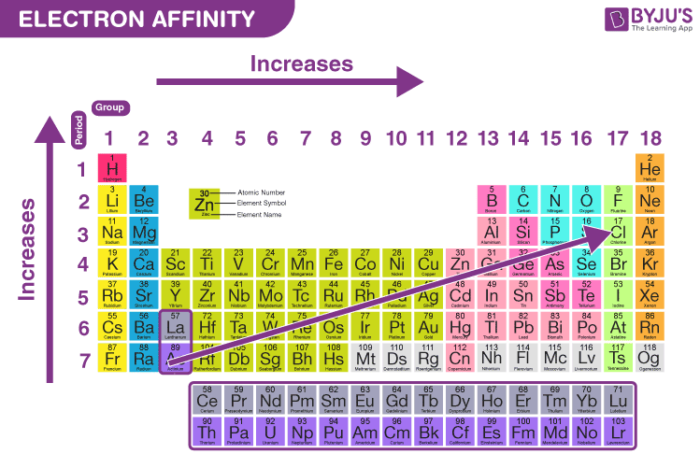
- General Trend: Electron affinity generally increases across a period (from left to right) and decreases down a group (from top to bottom) in the periodic table. This trend is attributed to the increasing effective nuclear charge (Zeff) across a period, leading to a stronger attraction for electrons. Conversely, the increasing atomic size down a group weakens the attraction between the nucleus and incoming electrons.
- Exceptions: Notable exceptions exist, particularly for elements with half-filled or completely filled electron shells. These configurations possess extra stability, leading to a lower EA than expected based on the general trend. For example, nitrogen (N) has a lower EA than oxygen (O) despite being located to its left on the periodic table.
- Noble Gases: Noble gases, with their filled valence shells, exhibit near-zero or even negative electron affinities. This indicates a strong reluctance to accept additional electrons due to the high stability of their electronic configuration.
2025: A New Era of Understanding
While the general trends remain valid, significant advancements in theoretical and experimental techniques have refined our understanding of electron affinity and revealed intricate nuances:
1. Relativistic Effects:
- Heavier Elements: As we move to heavier elements, relativistic effects become increasingly prominent. These effects, arising from the high speeds of electrons near the nucleus, influence electron energies and, consequently, electron affinity.
- Au and Hg: A striking example is the anomalous electron affinity of gold (Au) and mercury (Hg). Relativistic effects lead to a contraction of the 6s orbital, making it more difficult for Au and Hg to accept an electron. This explains why their EA values are lower than expected.
2. Quantum Chemical Calculations:
- Enhanced Accuracy: Advances in quantum chemical calculations have enabled highly accurate predictions of electron affinities. These calculations provide valuable insights into the electronic structure of atoms and ions, shedding light on the underlying factors influencing EA.
- Beyond the Periodic Table: Quantum chemical methods have expanded our ability to predict electron affinities for exotic species like superheavy elements and even molecules, pushing the boundaries of traditional periodic trends.
3. Experimental Techniques:
- High-Precision Measurements: Sophisticated experimental techniques, such as photoelectron spectroscopy and laser spectroscopy, have enabled unprecedented precision in measuring electron affinities. These advancements have refined our understanding of EA values and revealed subtle variations within groups and periods.
- Novel Systems: New experimental approaches are probing electron affinities in complex systems, including clusters, nanoparticles, and even single molecules. This research provides valuable insights into the role of electron affinity in controlling the properties of these systems.
Implications for the Future of Chemistry
The evolving understanding of electron affinity trends holds profound implications for various fields of chemistry:
1. Materials Science:
- Novel Materials Design: Electron affinity plays a crucial role in determining the electrical conductivity, optical properties, and reactivity of materials. By understanding and controlling electron affinities, researchers can design novel materials with tailored properties for specific applications, ranging from solar cells and batteries to catalysts and sensors.
- Nanomaterials: The electron affinity of nanomaterials is particularly important due to their high surface area-to-volume ratio. By tuning the electron affinity of nanomaterials, researchers can create materials with enhanced catalytic activity, improved charge transport properties, and unique optical properties.
2. Chemical Reactivity:
- Predicting Reaction Pathways: Electron affinity is a key factor in determining the likelihood and pathway of chemical reactions. Understanding electron affinity trends allows chemists to predict reaction products and optimize reaction conditions for desired outcomes.
- Catalysis: Electron affinity plays a crucial role in heterogeneous catalysis, where the adsorption and activation of reactants on the surface of a catalyst are influenced by the electron affinity of the catalyst material.
3. Atmospheric Chemistry:
- Ozone Depletion: Electron affinity plays a significant role in atmospheric chemistry, particularly in understanding the reactions involved in ozone depletion. The electron affinity of halogens, such as chlorine and bromine, influences their ability to participate in ozone-depleting reactions.
- Air Quality: Understanding the electron affinities of various pollutants and atmospheric constituents is crucial for developing strategies to mitigate air pollution and improve air quality.
4. Astrochemistry:
- Interstellar Molecules: Electron affinity plays a role in the formation and stability of interstellar molecules, which are observed in the vast expanse of space. By understanding electron affinity trends, astronomers can better interpret the spectral signatures of these molecules and gain insights into the chemical processes occurring in the interstellar medium.
- Exoplanet Atmospheres: Electron affinity is also relevant in the study of exoplanet atmospheres. The presence and abundance of certain elements, influenced by their electron affinity, can provide clues about the composition and evolution of these distant worlds.
Challenges and Future Directions
Despite the significant progress made in understanding electron affinity trends, several challenges remain:
- Complex Systems: Predicting electron affinities in complex systems, such as those involving multiple atoms, molecules, or surfaces, remains a significant challenge. Developing theoretical models and experimental techniques to address these complexities is crucial.
- Time-Dependent Effects: Electron affinity can vary with time, especially in dynamic systems. Understanding and predicting time-dependent electron affinity trends is essential for unraveling the complexities of chemical reactions and processes.
- Data Availability: A comprehensive database of accurate and reliable electron affinity values is crucial for advancing research in various fields. Efforts to expand and refine such databases are ongoing.
Conclusion
The study of electron affinity trends is a dynamic and evolving field. The advancements in theoretical models, experimental techniques, and computational methods have significantly enhanced our understanding of this fundamental property. As we move forward, the exploration of electron affinity will continue to drive innovations in materials science, chemical reactivity, atmospheric chemistry, and astrochemistry, paving the way for a deeper understanding of the world around us.