Demystifying The Periodic Trends: A Comprehensive Worksheet For 2025

Demystifying the Periodic Trends: A Comprehensive Worksheet for 2025
The periodic table is a cornerstone of chemistry, offering a beautifully organized framework to understand the behavior of elements. But beyond memorizing the symbols and atomic numbers, lies the fascinating world of periodic trends. These predictable patterns in the properties of elements are crucial for understanding chemical reactions, predicting the behavior of compounds, and even developing new materials.
This worksheet aims to equip you with the knowledge and tools to confidently navigate the periodic trends. It’s designed for students in 2025, incorporating the latest advancements in chemistry and addressing the ever-evolving understanding of these fundamental concepts.
1. Understanding the Basics:
- Atomic Number: This fundamental number defines an element, representing the number of protons in its nucleus. As we move across a period (row) from left to right, the atomic number increases by one, indicating the addition of a proton.
- Electron Configuration: The arrangement of electrons in different energy levels and orbitals determines the chemical behavior of an element. Understanding electron configuration is crucial for grasping periodic trends.
- Valence Electrons: These are the electrons in the outermost shell of an atom, responsible for forming chemical bonds and influencing reactivity.
- Effective Nuclear Charge: The net positive charge experienced by an electron in an atom is called the effective nuclear charge. It’s a crucial factor influencing the size of an atom and its tendency to attract electrons.
2. Periodic Trends: A Comprehensive Overview
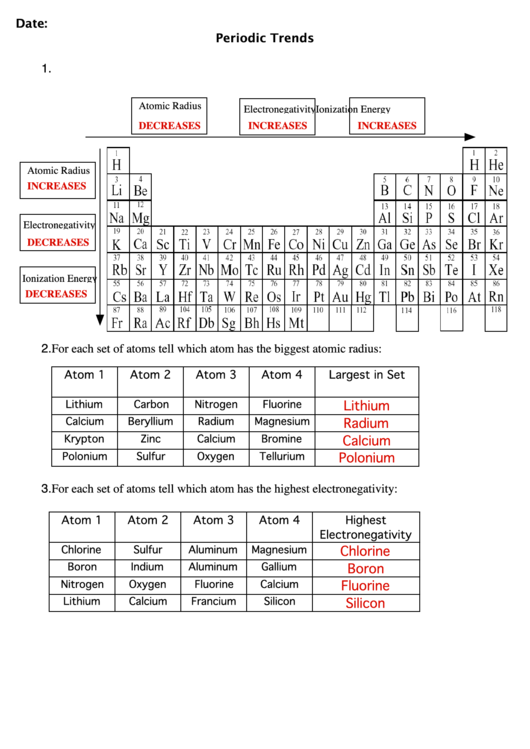
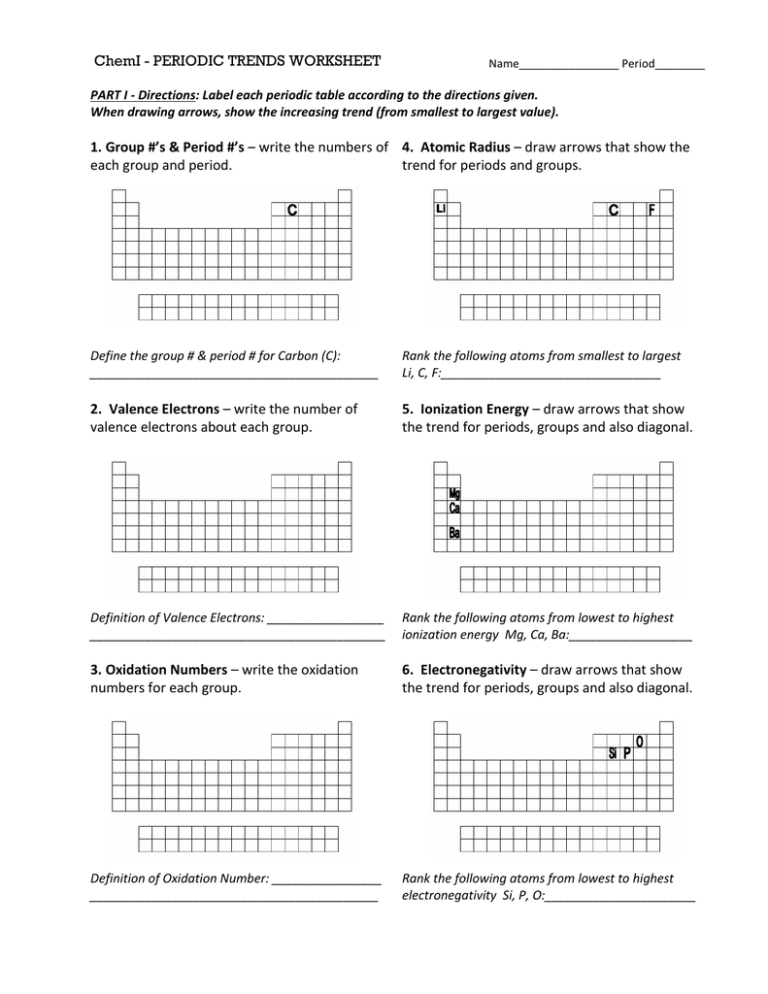
2.1. Ionization Energy:
- Definition: The minimum energy required to remove an electron from a gaseous atom in its ground state.
- Trend: Ionization energy generally increases from left to right across a period and decreases down a group.
- Explanation: As we move across a period, the effective nuclear charge increases, pulling the electrons closer to the nucleus, making it harder to remove an electron. Down a group, the outermost electron is further from the nucleus, experiencing weaker attraction, thus requiring less energy for removal.
- Exceptions: There are some exceptions to this trend due to electron configuration and the presence of half-filled and fully filled subshells. For instance, the ionization energy of nitrogen is higher than oxygen due to the stability of the half-filled p-orbital in nitrogen.
- Applications: Ionization energy helps understand the reactivity of elements. Elements with low ionization energies tend to lose electrons easily and form cations, while elements with high ionization energies tend to gain electrons and form anions.
2.2. Electron Affinity:
- Definition: The change in energy when an electron is added to a neutral gaseous atom to form a negative ion.
- Trend: Electron affinity generally increases from left to right across a period and decreases down a group.
- Explanation: As we move across a period, the effective nuclear charge increases, making the atom more attractive to an incoming electron. Down a group, the outermost electron is further from the nucleus, experiencing weaker attraction, thus resulting in lower electron affinity.
- Exceptions: Electron affinity values can be influenced by the electron configuration and the size of the atom. For instance, noble gases have a near-zero electron affinity due to their stable electron configurations.
- Applications: Electron affinity helps understand the tendency of an atom to gain electrons and form anions.
2.3. Electronegativity:
- Definition: The ability of an atom in a molecule to attract electrons towards itself.
- Trend: Electronegativity generally increases from left to right across a period and decreases down a group.
- Explanation: The same factors influencing ionization energy and electron affinity also affect electronegativity. Increased effective nuclear charge across a period makes the atom more attractive to electrons, while the increased distance of the outermost electron from the nucleus down a group reduces this attraction.
- Applications: Electronegativity is crucial for understanding the nature of chemical bonds. A large difference in electronegativity between two atoms results in an ionic bond, while a smaller difference leads to a covalent bond.
2.4. Atomic Radius:
- Definition: The distance between the nucleus of an atom and its outermost electron shell.
- Trend: Atomic radius generally decreases from left to right across a period and increases down a group.
- Explanation: As we move across a period, the effective nuclear charge increases, pulling the electrons closer to the nucleus, resulting in a smaller atomic radius. Down a group, the addition of electron shells leads to an increase in atomic radius.
- Applications: Atomic radius plays a significant role in determining the physical properties of elements, including their density and melting point. It also influences the strength and length of chemical bonds.
2.5. Metallic Character:
- Definition: The tendency of an element to lose electrons and form positive ions (cations).
- Trend: Metallic character generally increases down a group and decreases across a period.
- Explanation: Elements with low ionization energies tend to lose electrons easily, exhibiting strong metallic character. As we move down a group, the outermost electron is further from the nucleus, experiencing weaker attraction, making it easier to lose electrons.
- Applications: Metallic character helps understand the conductivity, malleability, and ductility of elements. Metals are excellent conductors of heat and electricity due to their ability to lose electrons easily.
3. Worksheet Activities:
3.1. Multiple Choice:
- Which of the following elements has the highest ionization energy?
a) Sodium (Na)
b) Chlorine (Cl)
c) Potassium (K)
d) Bromine (Br) - Which element has the largest atomic radius?
a) Lithium (Li)
b) Beryllium (Be)
c) Boron (B)
d) Carbon (C) - Which of the following elements has the strongest metallic character?
a) Fluorine (F)
b) Oxygen (O)
c) Cesium (Cs)
d) Nitrogen (N) - Which element has the highest electronegativity?
a) Oxygen (O)
b) Fluorine (F)
c) Nitrogen (N)
d) Chlorine (Cl) - Which element has the most negative electron affinity?
a) Sodium (Na)
b) Chlorine (Cl)
c) Potassium (K)
d) Bromine (Br)
3.2. True or False:
- Ionization energy generally decreases across a period.
- Atomic radius generally increases down a group.
- Elements with high electronegativity tend to lose electrons easily.
- Metallic character increases across a period.
- Electron affinity is the energy released when an electron is added to a neutral atom.
3.3. Short Answer:
- Explain the relationship between ionization energy and effective nuclear charge.
- Describe the trend in electronegativity across a period and down a group.
- What are the factors that influence the metallic character of an element?
- Why does atomic radius decrease across a period?
- How does electron configuration affect the ionization energy of an element?
3.4. Application Questions:
- Based on periodic trends, predict the relative ionization energies of sodium (Na) and potassium (K).
- Explain why fluorine (F) has a higher electronegativity than oxygen (O).
- Based on periodic trends, predict the relative atomic radii of lithium (Li) and beryllium (Be).
- Which element would you expect to have a more negative electron affinity: nitrogen (N) or oxygen (O)? Explain your reasoning.
- Describe the relationship between metallic character and the tendency of an element to form cations.
4. Advanced Concepts:
- Shielding Effect: The inner electrons shield the outer electrons from the full attraction of the nucleus, reducing the effective nuclear charge.
- Penetration Effect: The ability of an electron in a particular orbital to penetrate the electron cloud of inner electrons and come closer to the nucleus.
- Electron-Electron Repulsions: The repulsion between electrons in the same shell can influence the ionization energy and electron affinity.
- Relativistic Effects: At high atomic numbers, the speed of electrons becomes comparable to the speed of light, requiring relativistic corrections to accurately predict their behavior.
5. Conclusion:
Understanding periodic trends is crucial for any aspiring chemist. This worksheet serves as a stepping stone to explore the fascinating world of elements and their properties. By mastering these concepts, you’ll be equipped to analyze chemical reactions, predict the behavior of compounds, and even contribute to the development of new materials.
The periodic table is not just a static table of elements but a dynamic tool that allows us to understand the fundamental principles governing the behavior of matter. So, dive in, explore, and discover the wonders hidden within the periodic trends!
Note: This worksheet is designed to be a starting point for exploring periodic trends. It’s crucial to consult your textbook, class notes, and other resources for further understanding and a more comprehensive approach to this fascinating topic.