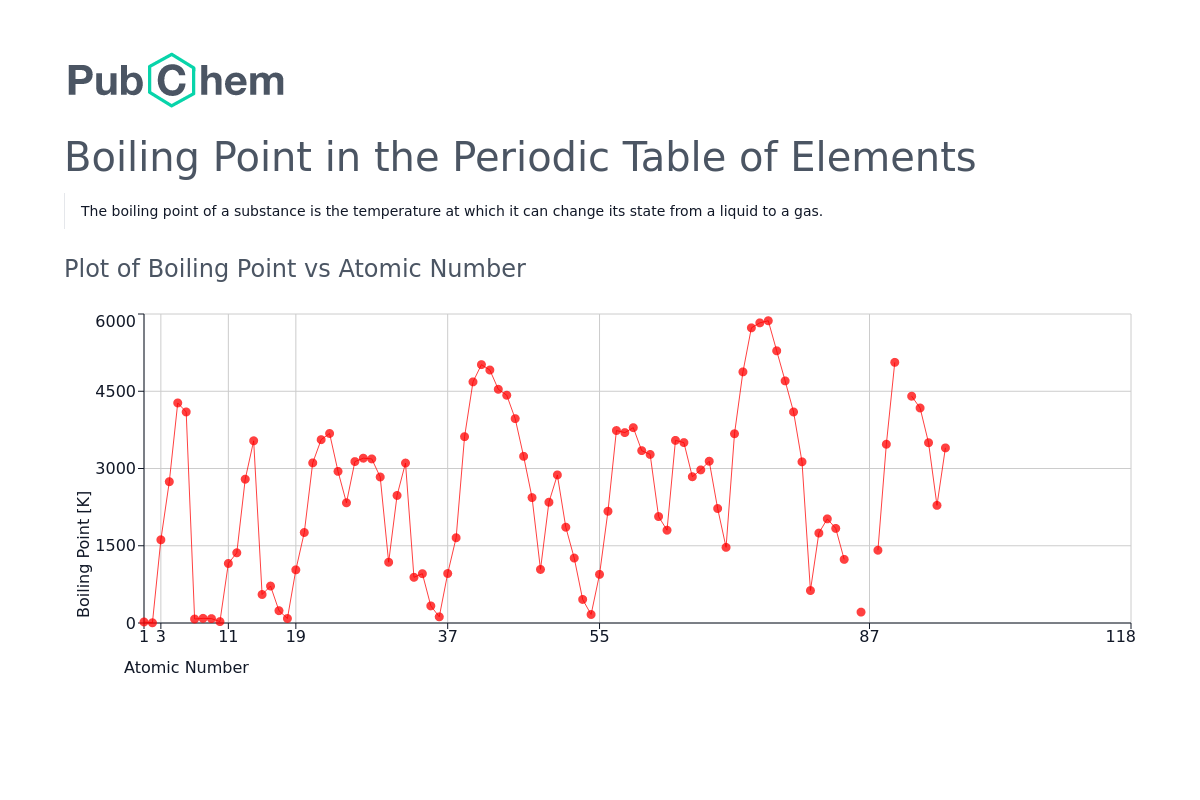
Boiling Point Trends In The Periodic Table: A 2025 Perspective

Boiling Point Trends in the Periodic Table: A 2025 Perspective
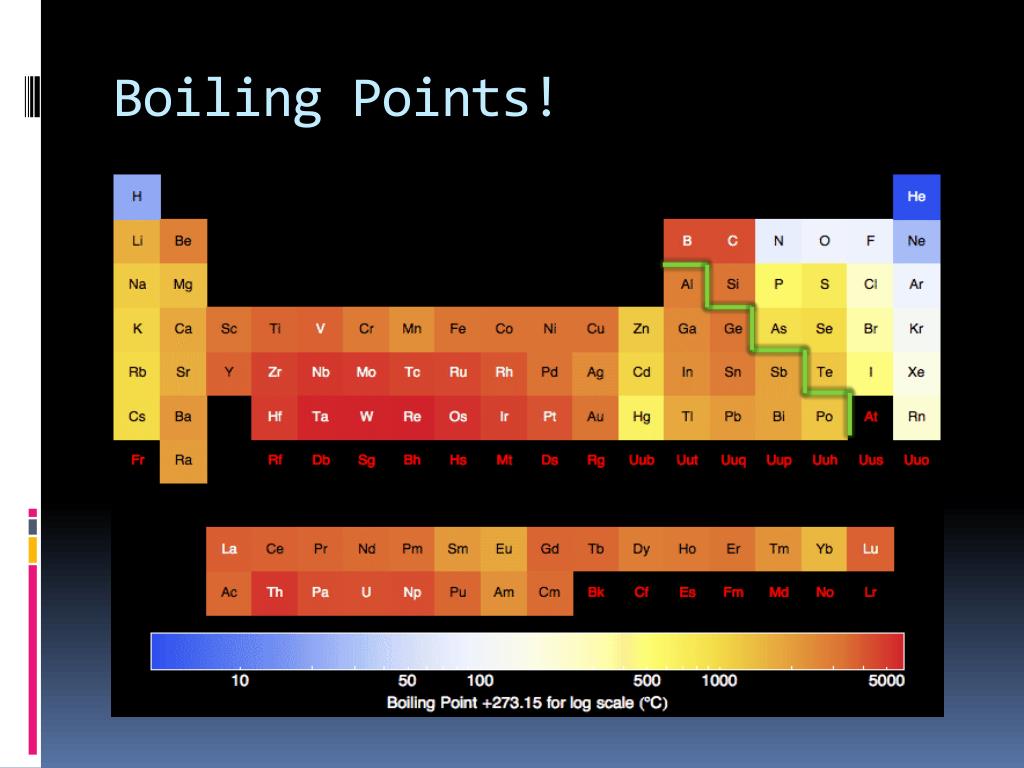
The periodic table, a masterpiece of scientific organization, reveals intricate patterns in the properties of elements. One such pattern, the boiling point trend, has fascinated chemists for centuries. As our understanding of interatomic and intermolecular forces evolves, so too does our interpretation of this trend. In 2025, armed with advanced computational tools and experimental techniques, we can delve deeper into the nuances of boiling point behavior across the periodic table.
The Basics: A Recap
Boiling point, the temperature at which a substance transitions from liquid to gas, is directly related to the strength of intermolecular forces. These forces, arising from the interactions between molecules, dictate the energy required to overcome them and enter the gaseous state.
- Metallic Bonding: Metals exhibit strong metallic bonding, characterized by a "sea" of delocalized electrons. This strong bonding leads to high boiling points, often exceeding 1000°C.
- Covalent Bonding: Non-metals form covalent bonds, sharing electrons to achieve stability. The strength of these bonds varies significantly, influencing boiling point. Larger, more complex molecules often exhibit higher boiling points due to increased van der Waals forces.
- Ionic Bonding: Ionic compounds, formed by electrostatic attraction between oppositely charged ions, generally have high boiling points. The strong electrostatic forces require considerable energy to overcome.
Beyond the Basics: A 2025 Perspective
While the fundamental concepts remain constant, our understanding of boiling point trends has significantly advanced in recent years.
1. The Role of Molecular Geometry and Polarity:
The shape of a molecule and its polarity play crucial roles in determining boiling point.
- Molecular Shape: Branching within a molecule reduces surface area available for intermolecular interactions, leading to weaker forces and lower boiling points. For example, straight-chain alkanes have higher boiling points than their branched isomers.
- Polarity: Polar molecules, possessing uneven electron distribution, exhibit stronger dipole-dipole interactions, contributing to higher boiling points compared to non-polar molecules. This is evident in comparing water (highly polar, high boiling point) to methane (non-polar, low boiling point).
2. The Influence of Hydrogen Bonding:
Hydrogen bonding, a particularly strong type of dipole-dipole interaction, significantly elevates boiling points. This force arises when a hydrogen atom is bonded to a highly electronegative atom (like oxygen, nitrogen, or fluorine).
- Water as an Example: Water’s high boiling point (100°C) is attributed to extensive hydrogen bonding networks. Each water molecule can form up to four hydrogen bonds with neighboring molecules, leading to a highly cohesive structure.
3. Computational Modeling: Predicting Boiling Points
Computational chemistry has revolutionized our ability to predict and understand boiling point trends. Sophisticated software programs, like Gaussian and Spartan, enable researchers to model the interactions between molecules and calculate their boiling points with remarkable accuracy.
- Density Functional Theory (DFT): DFT methods, based on the principles of quantum mechanics, provide a powerful framework for calculating electronic structure and intermolecular forces. These calculations can predict boiling points with high precision, even for complex molecules.
- Molecular Dynamics (MD) Simulations: MD simulations simulate the movement of molecules over time, allowing researchers to observe intermolecular interactions and predict boiling points based on the dynamics of the system.
4. Unveiling the Secrets of the Noble Gases:
The noble gases, once considered inert, have proven to be fascinating subjects in the study of boiling point trends.
- Van der Waals Forces: The weak van der Waals forces between noble gas atoms increase with atomic size. This trend is evident in the increasing boiling points down Group 18: Helium (4.2 K), Neon (27.1 K), Argon (87.3 K), Krypton (119.9 K), Xenon (165.0 K), and Radon (211.3 K).
- Challenges in Studying Radon: Radon’s high radioactivity poses significant challenges to experimental determination of its boiling point. However, computational methods have provided valuable insights into its behavior.
5. Boiling Point Trends in the Future: Emerging Applications
The study of boiling point trends is not merely an academic pursuit. It has far-reaching implications for various fields:
- Materials Science: Understanding boiling point trends is crucial for designing materials with specific properties, such as high thermal stability or low volatility. This knowledge finds applications in developing advanced ceramics, polymers, and high-performance lubricants.
- Chemical Engineering: Boiling point predictions are essential for optimizing distillation processes and designing efficient separation techniques. This knowledge is particularly important in the chemical industry, where separation and purification are crucial steps.
- Environmental Science: Boiling points influence the fate and transport of pollutants in the environment. Understanding these trends helps predict the volatility of hazardous substances and develop strategies for their safe disposal.
6. Beyond the Periodic Table: Boiling Point Trends in Non-Elemental Systems
The concept of boiling point extends beyond elemental substances. It applies to various mixtures and compounds, including solutions, alloys, and even biological systems.
- Solutions: The boiling point of a solution is influenced by the presence of dissolved solutes. This phenomenon, known as boiling point elevation, is directly related to the concentration of the solute.
- Alloys: The boiling points of alloys, mixtures of metals, can vary significantly depending on the composition and the interactions between the constituent metals. This understanding is crucial for designing alloys with specific melting and boiling points.
- Biological Systems: Boiling point trends are relevant to understanding the behavior of biological molecules, such as proteins and enzymes. The boiling point of water, for example, plays a critical role in maintaining the stability of biological systems.
7. Challenges and Future Directions
While our understanding of boiling point trends has advanced significantly, several challenges remain:
- Predicting Boiling Points of Complex Systems: Accurately predicting boiling points of complex mixtures, especially those containing multiple components with diverse interactions, remains a significant challenge.
- Understanding the Role of Quantum Effects: For very small molecules or at extremely low temperatures, quantum effects can influence boiling point behavior. Further research is needed to fully understand these phenomena.
- Developing Novel Experimental Techniques: New experimental techniques, such as high-throughput screening and microfluidics, are needed to efficiently measure boiling points of a wide range of materials.
Conclusion
The study of boiling point trends in the periodic table is a dynamic field, constantly evolving with advancements in computational tools and experimental techniques. As we delve deeper into the intricate interplay of interatomic and intermolecular forces, we gain a more profound understanding of this fundamental property. This knowledge, in turn, fuels innovations across various disciplines, from materials science to environmental science, shaping our future and driving scientific progress. The boiling point, a seemingly simple concept, reveals a complex and fascinating story of the interactions that govern the world around us.