Boiling Point Trends Across The Periodic Table: 2025 And Beyond

Boiling Point Trends Across the Periodic Table: 2025 and Beyond
The periodic table, a cornerstone of chemistry, provides a framework for understanding the properties of elements and their predictable behavior. One crucial property, boiling point, dictates when a substance transitions from a liquid to a gas. This seemingly simple concept holds profound implications for a vast array of scientific and technological applications, from materials science to astrophysics.
As we stand on the cusp of 2025, our understanding of boiling point trends across the periodic table has undergone a dramatic evolution, fueled by advancements in computational chemistry, experimental techniques, and a growing appreciation for the interplay of fundamental forces. This article delves into the intricate world of boiling point trends, exploring the factors that govern them, the latest discoveries, and the exciting avenues for future research.
The Fundamental Forces Shaping Boiling Point
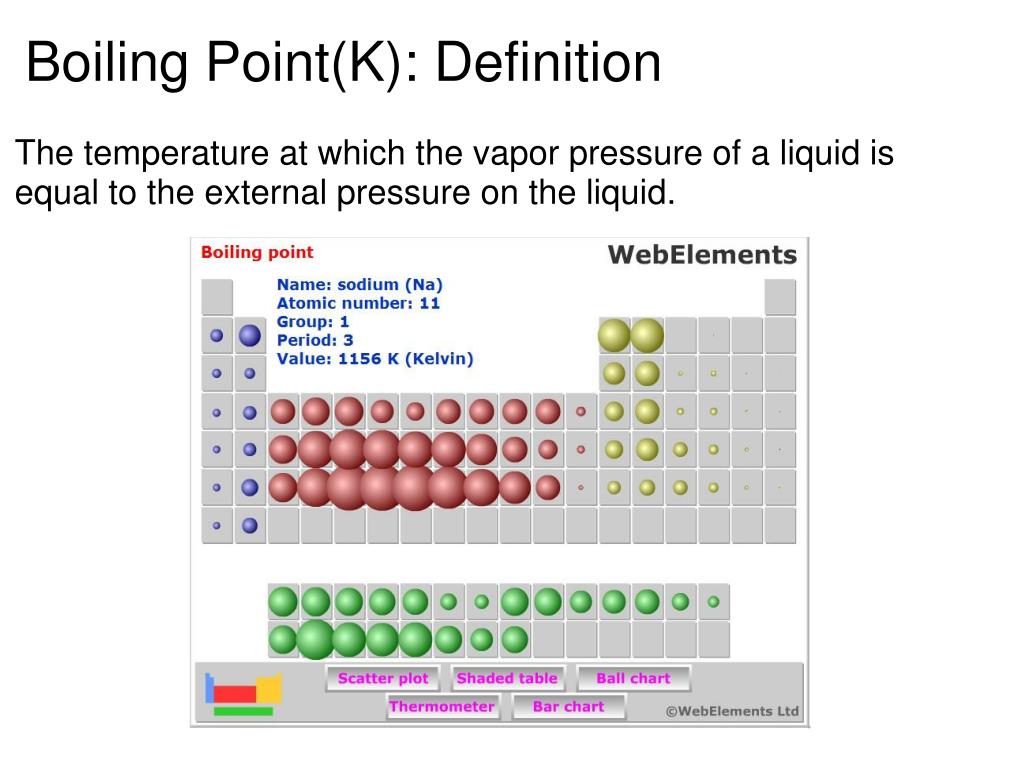
The boiling point of a substance is determined by the strength of intermolecular forces, the attractive forces that hold molecules together in the liquid state. These forces vary significantly across the periodic table, leading to the observed trends:
1. Intermolecular Forces: The Glue That Holds Liquids Together
- Van der Waals Forces: These weak, temporary attractions arise from temporary fluctuations in electron distribution within molecules. They are present in all substances but are strongest in large, polarizable molecules.
- Dipole-Dipole Interactions: These occur between polar molecules with permanent dipoles, resulting in stronger attractions compared to van der Waals forces.
- Hydrogen Bonding: This is the strongest type of intermolecular force, involving a special interaction between a hydrogen atom covalently bonded to a highly electronegative atom (like oxygen, nitrogen, or fluorine) and an electron pair on an adjacent molecule.
2. The Role of Molecular Size and Shape
Larger molecules have more electrons, leading to stronger van der Waals forces and higher boiling points. Similarly, more complex shapes, like those with branching or rings, can hinder close packing and weaken intermolecular forces, leading to lower boiling points.
3. The Impact of Metallic Bonding
Metals exhibit strong metallic bonding, where electrons are delocalized throughout the lattice. This results in high boiling points, as significant energy is required to overcome these strong attractions.
4. The Influence of Covalent Bonding
Covalent bonds within molecules are strong, but the intermolecular forces between covalent molecules are often weaker. This results in generally lower boiling points compared to metals.
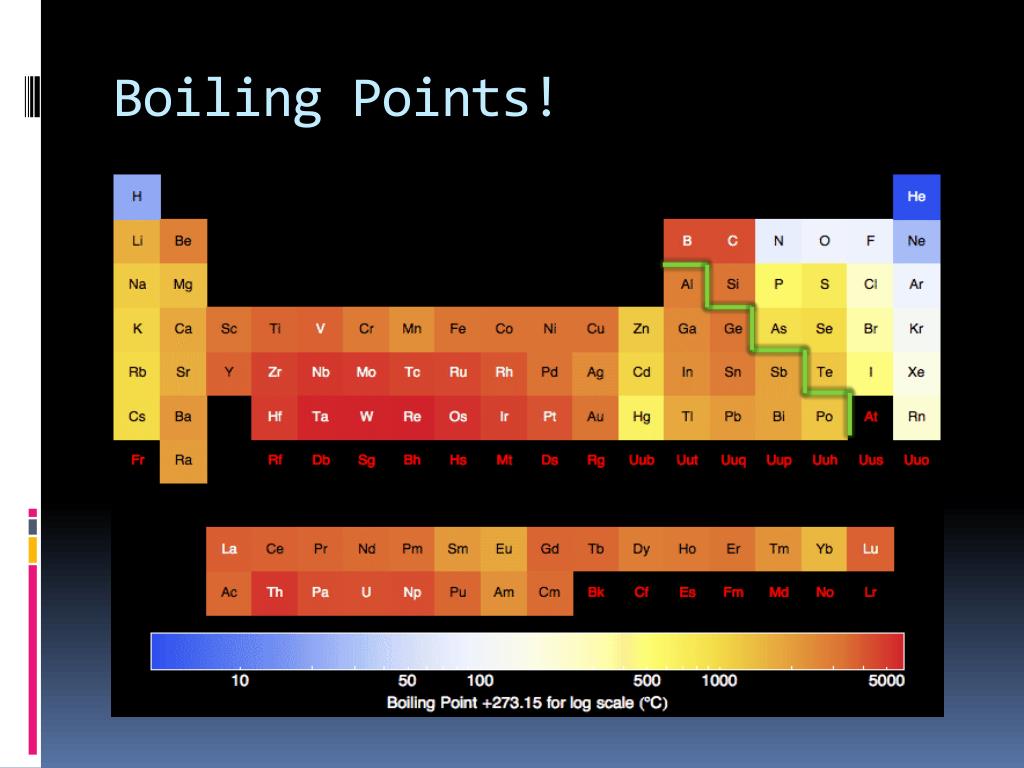
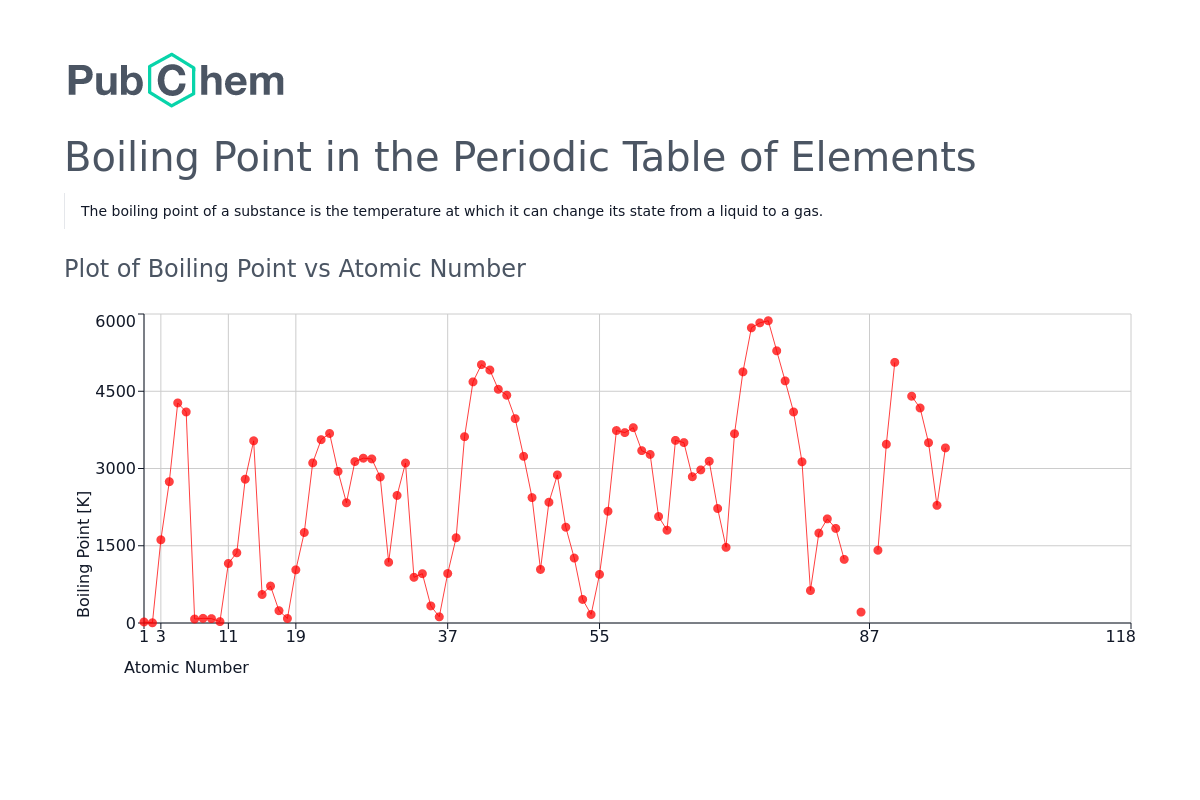
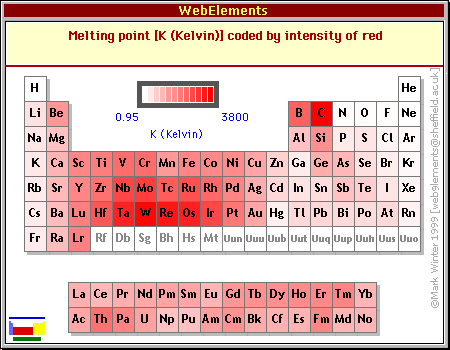
Boiling Point Trends Across Periods and Groups
The periodic table provides a powerful tool for predicting and understanding boiling point trends:
1. Across Periods:
- From Left to Right: Moving across a period, the number of electrons increases, leading to stronger van der Waals forces and generally higher boiling points. However, this trend is disrupted by the presence of non-metals, where weaker intermolecular forces dominate.
- Exceptions: Groups 14-17 exhibit a decrease in boiling point due to the increasing dominance of weaker intermolecular forces over van der Waals forces.
2. Down Groups:
- From Top to Bottom: Moving down a group, atomic size increases, leading to stronger van der Waals forces and higher boiling points. However, this trend is disrupted by the presence of hydrogen bonding, which can lead to higher boiling points for smaller molecules.
Recent Discoveries and Breakthroughs
In recent years, significant advances in computational chemistry and experimental techniques have revolutionized our understanding of boiling point trends:
1. Computational Modeling:
- Ab initio calculations: These highly accurate methods, based on first principles, have enabled precise prediction of boiling points for various molecules, even those not yet experimentally characterized.
- Molecular Dynamics simulations: These methods simulate the movement of molecules over time, allowing researchers to study the dynamics of boiling and gain insights into the factors governing boiling point.
2. Experimental Advancements:
- Laser-induced breakdown spectroscopy (LIBS): This technique uses laser pulses to vaporize materials and analyze their atomic composition, providing valuable information about boiling point and vaporization behavior.
- Microfluidic devices: These miniaturized systems allow for precise control of temperature and pressure, enabling the study of boiling point phenomena at smaller scales and under extreme conditions.
3. Uncovering the Role of Non-Covalent Interactions:
- π-π stacking: These interactions between aromatic rings are increasingly recognized as playing a significant role in determining boiling points, particularly in organic molecules.
- Hydrophobic interactions: These interactions between non-polar molecules can contribute significantly to boiling point, especially in systems involving water or other polar solvents.
The Future of Boiling Point Research
The field of boiling point research continues to evolve, with several exciting avenues for future exploration:
1. Understanding Boiling Point at Extreme Conditions:
- High pressure: Investigating how pressure affects boiling point is crucial for understanding processes in deep-sea environments and planetary interiors.
- Low temperature: Exploring the behavior of matter near absolute zero, where quantum effects become significant, offers a unique window into the nature of boiling.
2. Developing Novel Materials with Tailored Boiling Points:
- Designer solvents: Creating solvents with specific boiling points is crucial for various applications, from chemical synthesis to environmental remediation.
- Advanced materials: Understanding boiling point behavior is essential for designing materials with desired properties for applications in aerospace, energy storage, and electronics.
3. Exploring the Role of Boiling Point in Astrochemistry:
- Exoplanet atmospheres: Determining the boiling points of molecules in exoplanet atmospheres is key to understanding their composition and habitability.
- Interstellar medium: Understanding the boiling points of interstellar molecules helps us unravel the complex chemistry of the universe.
Conclusion
Boiling point, a seemingly simple property, reveals a rich tapestry of interactions and forces governing the behavior of matter. As we continue to push the boundaries of scientific understanding, the study of boiling point trends will remain a vital area of research, shaping our knowledge of the universe and enabling the development of new technologies that improve our lives. From the depths of the ocean to the vast expanse of space, the intricate dance of molecules as they transition from liquid to gas will continue to fascinate and inspire scientists for generations to come.



/periodictrendstable-5c4a46614cedfd000187c5db.jpg)